Transition Metals - Examples of Redox Reactions in Transition Metals (A-Level Chemistry)
Examples of Redox Reactions in Transition Metals
Reduction of Vanadate (V) ions
Metallic zinc is a good reducing agent.
It reacts with dilute acid to form Zn²⁺ ions and releases electrons for reduction.
Zn(s) → Zn²⁺(aq) + 2e⁻
Addition of zinc to vanadium(V) ions from a solution of ammonium vanadate (V) (NH₄VO₃) in acidic solution, will reduce the vanadium through each successive oxidation state. Each oxidation state produces a different colour.
Vanadium (V) ions are first reduced to vanadium (IV) ions:
2[VO₂(H₂O)₄]⁺(aq) + Zn(s) + 4H⁺(aq) → 2[VO(H₂O)₅]²⁺(aq) + Zn²⁺(aq)
Yellow Blue
Vanadium (IV) ions are then reduced to vanadium (III) ions:
2[VO(H₂O)₅]²⁺(aq) + Zn(s) +4H⁺(aq) → 2[V(H₂O)₆]³⁺+(aq) + Zn²⁺(aq)
Blue Green
Vanadium (III) ions are finally reduced to vanadium (II) ions:
Until now, the reactions have been feasible because their redox potentials have been positive.
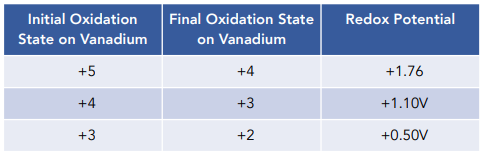
Vanadium (II) ions will however not be further reduced by zinc to vanadium metal because the redox potential for the reaction is negative, which means that the reaction is not feasible under standard conditions.
2[V(H₂O)₆]²⁺ (aq) + Zn(s) → V(s) + Zn²⁺(aq) + 6H₂O(l) E⦵ = -0.42V
Reduction of Silver Diammine [Ag(NH₃)₂]⁺
Silver diammine [Ag(NH₃)₂]⁺ is used in Tollen’s reagent to distinguish between aldehydes and ketones.
Aldehydes are strong enough reducing agents to reduce the silver (I) to metallic silver.
2[Ag(NH₃)₂]⁺ + CH₃CHO + H₂O → 2Ag(s) + 4NH₃ + CH₃COOH + 2H⁺
Ketones are unable to do this and have no reaction.
Reduction and Oxidation of Chromium Ions
The large number of oxidation states that can be taken by chromium, allow its to part-take in a wide variety of redox reactions.
When added to an acidic solution of zinc metal, dichromate (VI) ions are reduced to chromium (III) ions (E⦵ = +2.09V):
Cr₂O₇²⁻(aq) + 3Zn(s) + 14H⁺(aq) → 2Cr³⁺(aq) + 3Zn²⁺(aq) + 7H₂O(l)
Orange Green
Zinc will further reduce Cr³⁺ to Cr²⁺ (E⦵ = +0.35V):
2Cr³⁺(aq) + 3Zn(s) → 2Cr²⁺(aq) + Zn²⁺(aq)
Green Yellow
When added to an alkaline solution of hydrogen peroxide, chromium (III) ions are oxidised to chromate (IV) ions (E⦵ = +1.08V):
2Cr³⁺(aq) + 3H₂O₂(s) + 10OH⁻ (aq) → CrO₄²⁻(aq) + 8H₂O(l)
Green Yellow
If we then add some dilute sulphuric acid to the solution, Cr₂O₇²⁻ ions form turning the solution orange.
CrO₄²⁻(aq) + 2H⁺(aq) ⇌ Cr₂O₇²⁻(aq) + H₂O(l)
Yellow Orange
This reaction is reversible and an equilibrium between CrO₄²⁻ and Cr₂O₇²⁻ ions sets up.





Still got a question? Leave a comment
Leave a comment