Thermodynamic - Enthalpy of Solution (A-Level Chemistry)
Enthalpy of Solution
Dissolving Compounds
Enthalpy Change of Solution
Enthalpy change of solution, ΔsolH is the enthalpy change when one mole of an ionic compound is dissolved in a solvent to form an infinitely dilute solution, where the addition of further solvent does not produce further enthalpy change. The ions are well separated and do not interact with each other.
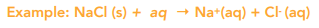
When a solute dissolves in a solvent the following happens:
- Bond breaking – Bonds between solute molecules break. This is an endothermic process.
- Bond formation – New bonds form between the solute and solvent molecules. This process is exothermic.
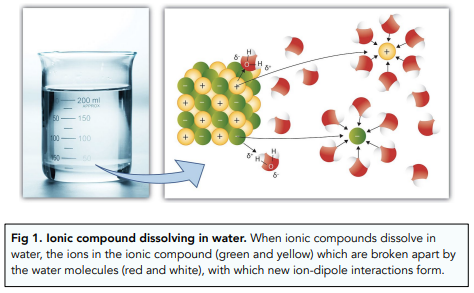
The overall enthalpy change of these two processes is the enthalpy change of solution.
For a solute to dissolve the energy given out has to be the same or greater than the energy taken in. Therefore, soluble compounds will always have a negative enthalpy change of solution and the more soluble the compound, the more negative its enthalpy change of solution.





Still got a question? Leave a comment
Leave a comment