Thermodynamic - Total Entropy Changes (A-Level Chemistry)
Total Entropy Changes
Total Entropy Changes
Key Terms
In a chemical reaction, the system refers to the reactants and products under investigation
The surroundings refer to anything other than the reactants and products. This can include the solvent, the air around the container in which the reaction is taking place, the container itself, and anything that is dipped into the reacting mixture.
Entropy Change of the Surroundings
During a chemical reaction, energy is transferred between the system and the surroundings in the form of heat, so that the entropy of both the system and the surroundings gets altered.
In exothermic reactions, energy gets transferred to the surrounding so that its entropy is likely to increase.
In endothermic reactions, energy gets absorbed from the surroundings so that its entropy is likely to decrease.
The formula for calculating ΔSsurroundings is as follows:
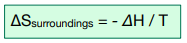
Where ΔH represents enthalpy change of the reaction in kJ mol-1 and T represents temperature in K.
Total Entropy Change
Total entropy change combines the entropy change of the system and the entropy change of the surroundings.
The formula for calculating ΔStotal is as follows:

Worked example: Calculate the total entropy change for the following reaction at 300K:

1. Calculate the entropy change of the surroundings using the equation. For this you will need the enthalpy change of the reaction and the temperature. Bear in mind that enthalpies are measured in kJ but entropies have to be in J. You will therefore have to convert values in kJ to J before doing any calculations.
ΔH = -65 kJ mol-1 x 1000 = -65000 J mol-1
ΔSsurroundings = – ΔH / T
ΔSsurroundings = – (-65000 J mol-1) / 300 K = – 593.3 J K-1 mol-1
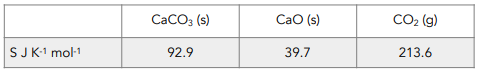
2. Calculate the entropy of the system using the equation. Subtract the total entropy of the products (what you end up with) from the total entropy of the reactants (what you started with).
∑Δ S (products) = S (CaO) + S (CO2)
= 39.7 + 213.6
= 253.3 J K-1 mol-1
∑Δ S (reactants) = S (CaCO3)
= 92.9 J K-1 mol-1
ΔSsystem =∑Δ S (products) – ∑ΔS (reactants)
ΔSsystem = 253.3 – 92.9 = +160.4 J K-1 mol-1
3. Calculate total entropy change using the equation. Simply substitute the previously calculated values into the equation.
ΔStotal = ΔSsystem + ΔSsurroundings
ΔStotal = 160.4 + (-593.3) = -433 J K-1 mol-1 (to 3 s.f.)
Feasible Reactions
A feasible reaction is a reaction that is likely to occur when reactants are mixed and the activation energy is not getting in the way.
- Reactions with a negative ΔH are more likely to be feasible but it is not a requisite. Many exothermic reactions will occur spontaneously, because they do not require an external energy source (i.e. they give off energy, not take it in). A negative ΔH is a factor that contributes to whether a reaction will occur on its own accord, however it doesn’t explain why many endothermic reactions (ΔH is positive), also occur spontaneously. Something else must be happening.
- Reactions with a positive ΔSsystem are more likely to be feasible but it is not guaranteed. This is because the natural movement of a system is towards the state of greater stability, this is, of greater entropy.
- For a reaction to be feasible its total entropy change has to be positive. Unlike ΔSsystem , total entropy change takes into account the role played by enthalpy, temperature and kinetics on determining whether a reaction will occur. If any of this factors changes, so will the value for ΔStotal and hence the feasibility of the reaction.
Worked example: Is the following reaction feasible at a) 300K, b) 1200K?
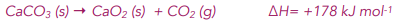


Answer:
As we saw in the previous worked example, at 300K ΔStotal = -433 K-1 mol-1 (to 3 s.f.). This is a negative value so the reaction is not feasible at 300K.
If we repeat the calculations for ΔStotal this time at T=1200K, we get that:
ΔSsurroundings = – (-65000 J mol-1) / 1200 K = – 148.3 J K-1 mol-1
ΔSsystem = Stays the same = +160.4 K-1 mol-1
ΔStotal = 160.4 + (-148.3) = +12.1 K-1 mol-1 (to 3 s.f.)
At 1200K the value for ΔStotal is positive so the reaction is feasible at that temperature.





Still got a question? Leave a comment
Leave a comment