Aldehydes and Ketones - Testing for Carbonyl Compounds (A-Level Chemistry)
Testing for Carbonyl Compounds
Detecting the presence of carbonyl compounds
The presence of a compound with a carbonyl functional group can be detected from its reaction with two different reagents: 2,4- dinitrophenylhydrazine and alkaline iodine solution.
2,4-dinitrophenylhydrazine (2,4-DNPH)
When an aldehyde or a ketone is added to an acidic solution of 2,4- DNPH in methanol, a bright orange precipitate forms.
The reaction between the aldehyde/ketone and 2,4-DNPH is a condensation reaction. A water molecule is lost in the process.
The recrystallised precipitate can be used to identify the the exact carbonyl compound present by referring its melting point to melting point data bases.
Carboxylic acids, although they have carbonyl functional group too, do not form a precipitate.
Alkaline Iodine
When a compound with a CH₃CO⁻ group, such as methyl ketones and ethanal, are heated with alkaline iodine a yellow precipitate of triiodomethane forms, accompanied by an antiseptic smell.
The reaction takes place in two steps:
1. Halogenation of the methyl group. The three hydrogen atoms in the -CH3 group get replaced by iodine atoms.
2. Alkaline hydrolysis. The iodinated intermediate gets hydrolysed by the alkali in solution to release a triiodomethane molecule (CHI₃) and form a carboxylate salt.
The overall reaction looks as followed:
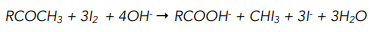
This test also gives a positive result for certain alcohols, namely those that be oxidised to give a carbonyl compound with a CH₃CO⁻ group. These include:
- Primary Alcohols – Only ethanol gives a positive test result. It is oxidised by the NaOH and iodine in solution to give ethanal.
- Secondary Alcohols – Secondary alcohols with a methyl group attached to the hydroxyl carbon will give a positive test result. They are oxidised by the NaOH and iodine in solution to give methyl ketones.
- Tertiary alcohols – Tertiary alcohols cannot be oxidised and hence no tertiary alcohol will give a positive test result.
Distinguishing between aldehydes and ketones
Secondary alcohols can be oxidised to make ketones. Ketones, however, cannot be further oxidised. This fact is the basis of the two chemical tests which distinguish aldehydes from ketones: The Fehling’s test and Tollen’s reagent.
Fehling’s test
Fehling’s solution is a blue solution which includes copper(II) ions dissolved in sodium hydroxide.
- Aldehydes – When an aldehyde is warmed with Fehling’s solution, the blue solution forms a brick-red precipitate of copper(I) oxide. The Cu²⁺ ions oxidise the aldehyde to the respective carboxylic acid. The aldehyde in turn reduces the Cu²⁺ ions to Cu⁺ ions.

- Ketones – There is no reaction with ketones.
Tollen’s reagent (silver mirror test)
Tollen’s reagent contains the complex ion silver diammine [Ag(NH₃)₂]⁺.
- Aldehydes – When an aldehyde is warmed with Tollen’s reagent in a clean test tube, metallic silver is formed around the inside of the test tube.

- Ketones – There is no reaction with ketones.
Aldehydes and ketones are a class of organic compounds that contain a carbonyl functional group (C=O). They are important in a variety of industrial and biological processes and are used as intermediates in the synthesis of more complex compounds.
The main difference between aldehydes and ketones is the position of the carbonyl group. In aldehydes, the carbonyl group is at the end of the carbon chain, while in ketones it is in the middle of the carbon chain.
Testing for carbonyl compounds is important in order to identify the presence of aldehydes and ketones in a sample, which is essential in the identification and characterization of compounds in the field of chemistry.
The common tests used to identify carbonyl compounds include the Tollens’ test, Fehling’s test, and Benedict’s test. These tests use different reagents to react with the carbonyl group and produce characteristic results, such as a silver mirror or a color change.
Tollens’ test works by reacting a sample of the unknown compound with Tollens’ reagent, which is a solution of silver nitrate and sodium hydroxide. If the sample contains an aldehyde, it will react with the silver ions in the reagent to form a silver metal, which will form a characteristic silver mirror on the inner surface of the test tube.
Fehling’s test works by reacting a sample of the unknown compound with Fehling’s solution, which is a mixture of copper(II) sulfate and sodium hydroxide. If the sample contains aldehydes or ketones, it will react with the copper ions in the solution to produce a brick-red precipitate, indicating the presence of a carbonyl group.
Benedict’s test works by reacting a sample of the unknown compound with Benedict’s reagent, which is a solution of copper(II) sulfate and sodium citrate. If the sample contains aldehydes or ketones, it will react with the copper ions in the solution to produce a characteristic color change, ranging from yellow to green to blue to brown, depending on the amount of reducing sugars present in the sample.






Still got a question? Leave a comment
Leave a comment