Equilibrium Constant for Homogenous Systems - Le Chatelier’s Principle in Gas Equilibria (A-Level Chemistry)
Le Chatelier’s Principle in Gas Equilibria
The Effect of a Changing Environment on a Gaseous Equilibrium
Le Chatelier’s principle states that any change to a system in equilibrium will cause the system to favour the reaction which will oppose the change.
Changes in pressure
Changes in pressure in gaseous equilibrium apply in the same way as changes in concentration apply to solution equilibrium.
- Increasing the pressure – causes equilibrium to shift to favour the direction with the fewer moles.
- Decreasing the pressure – causes equilibrium to shift to favour the direction with the most moles.
For example:
In the reaction to make ammonia
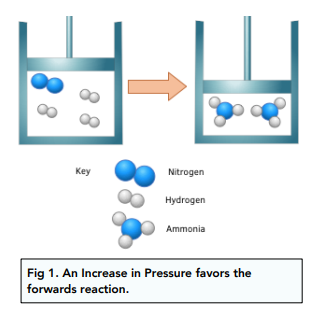
An increase in pressure will favour the forward reaction. This is because there are fewer moles and will result in a decrease in pressure.
Changes in temperature
Changes in temperature in gaseous equilibrium apply in the same way it applies to solution equilibrium:
- Increasing the temperature – causes equilibrium to shift in the endothermic direction, where ΔH is positive.
- Decreasing the temperature – causes equilibrium to shift in the exothermic direction, where ΔH is negative.
Worked example: Hydrogen is made in the following reaction:
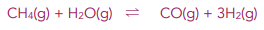
Explain what the effect is on the yield of hydrogen if the total pressure at equilibrium is reduced
Answer: The yield will increase (1).
To oppose the reduction in pressure, and cause the pressure to increase (1), the equilibrium will move to the side with most moles. This is the forward reaction (1)





Still got a question? Leave a comment
Leave a comment