Alkenes - Alkene Structure and Reactivity (A-Level Chemistry)
Alkene Structure and Reactivity
Alkene Structure
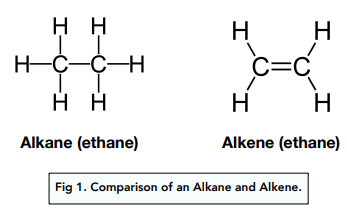
Alkenes are a group of compounds with the general formula CnH2n which each have a carbon-carbon double bond.
The presence of a C=C double bond makes alkenes unsaturated compounds.
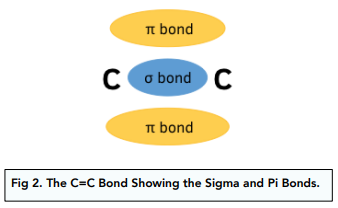
The C=C double bond consists of an overlap of electrons in the 2s subshell to form a sigma bond σ, plus the overlap of electrons from the 2p subshell results in a pi bond π .
The pi bond extends above and below the plane of the C atoms, resulting in no free rotation of the groups about the C atoms. This gives rise to E-Z isomerism in alkenes.
Also the C=C bond forms a high density of electrons.
Addition Reactions of Alkenes
Key Terms
Electrophiles are electron-pair acceptors, which means they accept electrons from other compounds such as alkenes. The electrophiles are attracted to regions of high electron density e.g. the C=C double covalent bond. Examples of electrophiles are either positively charged ions or polar molecules.
A carbocation is an ion with a positively charged carbon atom.
Electrophilic Addition Mechanism
- Alkenes undergo electrophilic addition reactions as they have double bonds. The C=C double bond is a region of high electron density which makes alkenes very reactive and very susceptible to attack by electrophiles
- In an electrophilic addition reaction of an alkene, the C=C double bond is broken. When alkenes undergo an electrophilic reaction, the double bond between the two carbons breaks and other atoms bond to the carbon atoms. Remember carbon is able to make up to 4 bonds as it has 4 electrons in its outermost electron shell.
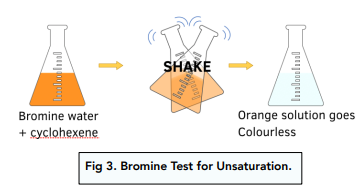
Bromine Test for Unsaturation
- Bromine water decolourises if an alkene is present. Bromine water can be used as a test for unsaturation as it changes from orange to colourless when unsaturated compounds (alkenes) are present. The alkene needs to be mixed or shaken with the bromine water solution.
- An electrophilic addition reaction occurs when bromine water reacts with alkenes. When an alkene reacts with bromine water, a dibromoalkane is formed which means two bromine atoms are added to each of the carbons when the double bond is broken. The dibromoalkane that forms is colourless.
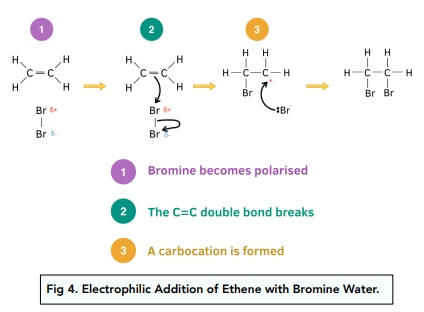
Electrophilic Addition of Ethene with Bromine Water
General equation: ![]()
- Bromine becomes polarised. As the C=C double covalent bond is a region of high electron density, it repels the electrons that are present in the Br2 molecule. As a result of this repulsion, the electrons in the bromine molecule are displaced to one side which causes Br2 to become polarised.
- The C=C double bond breaks. The bromine atom with a delta positive charge is attracted to the region of high electron density of the double bond. This causes a bond to be formed between this delta positive bromine atom and one of the carbon atoms in the double bond, causing the double bond to break.
- A carbocation is formed. A carbocation is formed which is a positively charged intermediate on the carbon atom that has not yet formed a bond. The bromine ion with a negative charge approaches the carbocation and forms a bond with it by donating its lone pair of electrons.
Electrophilic Addition of Alkenes with HBr
An electrophilic reaction takes place when alkenes react with hydrogen halides.
- Sometimes there may be an unsymmetrical alkene in the addition reaction. An unsymmetrical alkene is when the group of atoms attached to each carbon in the double bond are different.
- Propene is an example of an unsymmetrical alkene. Propane is unsymmetrical as it has two hydrogens bonded to one of the carbon atoms and then a hydrogen atom and methyl group (CH3) attached to the other carbon atom in the double bond.
- When an unsymmetrical alkene reacts with a hydrogen halide two products are formed. The product that forms in higher quantities is known as the major product and the one that forms in lower quantities is known as the minor product.
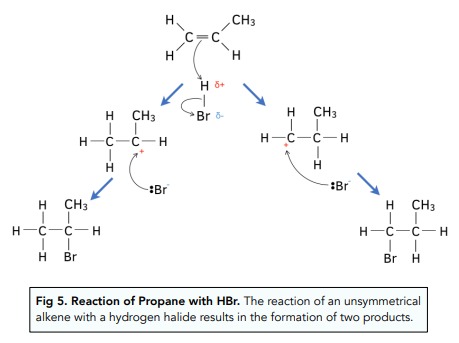
- Major and minor products can be determined using Markownikoff’s rule. Markownikkoff’s rule states that the major product will have the hydrogen in the hydrogen halide bond to the carbon atom already bonded to the highest number of hydrogens.
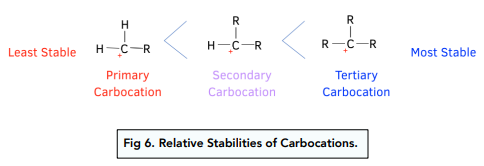
- The dominating product depends on the stability of the carbocation that is formed. During the reaction a carbocation intermediate will be formed and it is more stable when it has more alkyl groups (alkane with a hydrogen removed e.g. -CH3) attached to it. This is because the alkyl groups donate electrons to the positive charge of the carbocation making it more stable. Alkyl groups are said to have a positive inductive effect on the positively charged carbon atom.
Electrophilic Addition of Propene with Sulphuric Acid
- An electrophilic addition reaction takes place when alkenes react with sulphuric acid. When alkenes react with cold concentrated sulphuric acid it causes the production of alkyl hydrogen sulphates.
- The sulphuric acid acts as a catalyst in the reaction. As the sulphuric acid is still present at the end of the reaction, it does not get used up and therefore it acts as a catalyst.
- Again, if the reaction takes place with an unsymmetrical alkene, two products form. The major product depends on the formation of the most stable carbocation during the reaction.
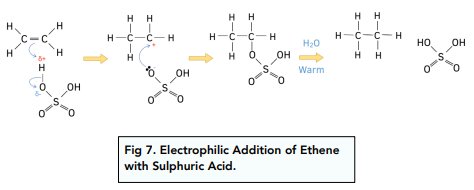
Electrophilic Addition of Ethene with Sulphuric Acid
Overall equation: ![]()
Step 1: The reaction of cold concentrated sulphuric acid with ethene which is the electrophilic addition
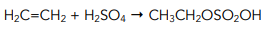
Step 2: Hydrolysis of the ethyl hydrogen sulphate by warming mixture with water to form ethanol.
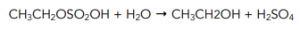
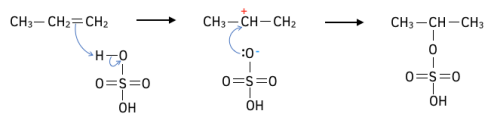
Worked Example: Propene reacts with sulfuric acid to form two isomers. Outline the reaction mechanism to show the formation of the major isomer.
Answer:
Other Addition Reactions
- Alkenes react with hydrogen in the presence of a metal catalyst to form an alkane. Hydrogen gets added across the C=C double bond in the presence of a nickel or platinum catalyst to form a saturated hydrocarbon. This mechanism is the basis behind the manufacture of margarine by catalytic hydrogenation of unsaturated vegetable oils.
- Alkenes react with steam to produce alcohols. This reaction takes place in the presence of an acid catalyst (such as phosphoric(V) acid), high temperatures (300ºC) and pressures (60 – 70 atm). The partial positive charge on the hydrogen atom in water molecules allows them to act as electrophiles. Ethanol can be synthesised from ethene this way.
Oxidation of alkenes
- Alkenes can be oxidised by potassium(VII) manganate (KMnO4). The products of oxidation will depend on the conditions of the reaction.
- Alkenes react with cold dilute acidified potassium manganate (VII) to form a diol. A diol is a compound with two -OH groups. The potassium manganate solution will change colour from purple to colourless.
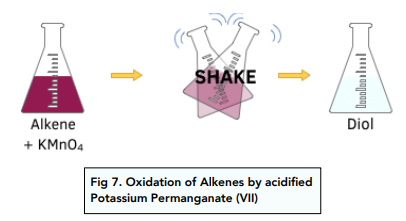
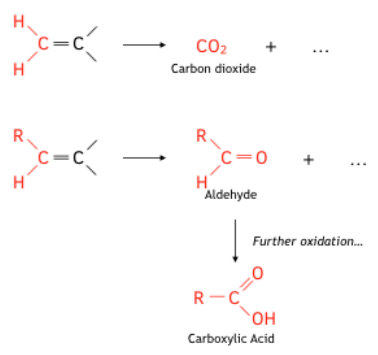
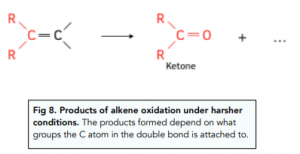
- Under hasher conditions the C=C double bond breaks completely. When alkenes react with hot, concentrated manganate (VII) solution the -OH groups in the initially formed diol get further oxidised to form either carbon dioxide, carboxylic acids, aldehydes or ketones, depending on the position of the C=C bond in the alkene molecule.
Alkenes are a type of organic molecule made up of carbon and hydrogen atoms. They are characterized by the presence of a double bond between two carbon atoms.
An alkene molecule consists of a carbon-carbon double bond and two single bonds to either hydrogen or other carbon atoms. The double bond allows the molecule to have different properties than other organic molecules, such as alkanes.
Alkenes are reactive due to the presence of the double bond between two carbon atoms. This bond is a source of reactivity because it is electron deficient and readily participates in chemical reactions.
Alkenes are more reactive than alkanes due to the presence of the double bond. The double bond is more electron deficient than a single bond, making it more susceptible to chemical reactions.
The most common reactions of alkenes include addition reactions, such as hydrogenation and halogenation, as well as elimination reactions, such as dehydration and alkene polymerization.
Alkene addition reactions occur through a process called electrophilic addition, in which an electrophile (an electron-deficient species) reacts with the alkene double bond, adding the electrophile to both carbon atoms of the double bond.
Alkene elimination reactions occur through a process called elimination, in which the double bond is broken and two new single bonds are formed. This reaction is the reverse of an addition reaction and typically results in the formation of a smaller molecule.
Alkene reactions have many real-life applications, including the production of plastics, synthetic rubber, and detergents. Alkenes are also used as precursors to many other chemicals and pharmaceuticals.
Understanding alkene structure and reactivity is important for A-Level Chemistry students because it provides a foundation for further studies in organic chemistry and biochemistry. Alkenes are important molecules with a wide range of applications, and understanding their structure and reactivity is crucial for a comprehensive understanding of chemistry.






Still got a question? Leave a comment
Leave a comment