Amino Acids, Proteins and DNA - Structure of Amino Acids (A-Level Chemistry)
Structure of Amino Acids
Amino Acids
All proteins are made up from amino acids.
Amino acids have two different functional groups attached to a chiral carbon, a carboxylic acid group (-COOH) and an amine group (-NH₂).
They also have an ‘R’ group which is the variable group, this is different for each amino acid.
The general formula of an amino acid is shown in the image below:
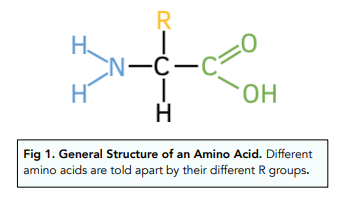
All amino acids, except glycine, contain a chiral carbon atom.
They show optical isomerism, as they form isomers which are mirrorimages of each other.
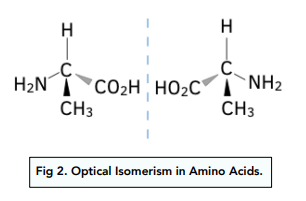
The isomers can be identified as they will rotate plane polarised light in opposite directions, to an equal extent.
Zwitterions
In an amino acid molecule, the carboxylic acid part of the molecule can lose a proton and act as an acid.
The amine group can gain a proton and act as a base.
For this reason, amino acids exist as structures known as zwitterions.
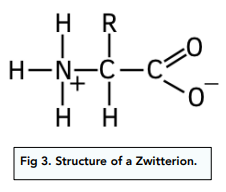
Zwitterions have both permanent positive charge and permanent negative charge, as shown in the diagram. Zwitterions therefore have a neutral charge overall.
In solution, zwitterions exist at their own unique pH.
Due to their opposite charges, zwitterions have strong electrostatic forces of attraction between them. This accounts for the following properties:
• Mostly exist in the form of a crystalline white solid at room temperature
• Have high melting points.
• Dissolve well in water
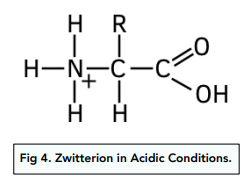
Structure of Zwitterions in Acidic Conditions
In strongly acidic conditions the amine group (NH₂) accepts a proton to form a positive ion.
The amine group is protonated as the lone pair of electrons on the nitrogen accepts a proton.
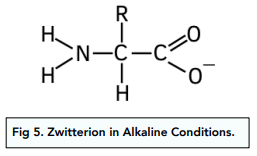
Structure of Zwitterions in Alkaline Conditions
In strongly alkaline conditions the carboxylic acid group (COOH) loses a proton to form a negative ion.
The carboxylic acid group has become deprotonated as the -OH group has lost a proton.
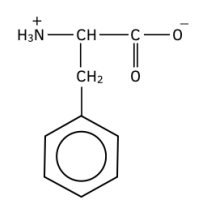
Worked example: Draw the zwitterion produced from phenylalanine
Answer:
Amino Acid Electrophoresis
Electrophoresis
Electrophoresis is an analytical method used to separate, identify and purify a protein or amino acid sample.
1. Set up the electrophoresis machine. The electrophoresis machine consists of a positive electrode (the anode) and a negative electrode placed at either end of a gel bathed in buffer solution.
Small amounts of the sample being investigated are pipetted into tiny wells cut at the centre of the gel.
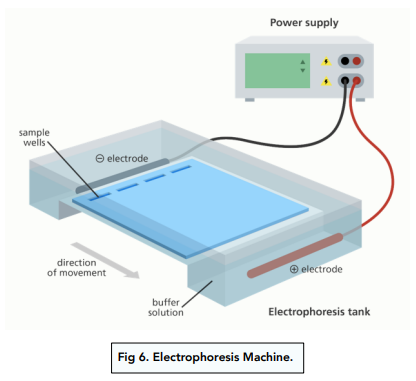
2. Apply an electric current. When an electric current is applied across the gel, the charged amino acids in the sample will migrate through the gel towards the opposite charged cathode.
3. Visualise the results – As the current is passed across the gel, different amino acids will migrate at different speeds. The amino acids will separate and stain to form a series of bands known as an electropherogram, which can be used to identify the amino acids present.
Amino Acid Separation
Amino acids or polypeptides being investigated will be effectively separated by electrophoresis according to their:
• Charge – Charged amino acids will migrate towards the oppositely charged electrode. The greater the charge on the amino acid, the faster they will migrate across the gel.
• Size – The larger, heavier the amino acid, the more slowly it will migrate across the gel.
Isoelectric Point
The isolelectric point is the pH value of an aqueous solution of an amino acid.
As amino acids are amphoteric, when they are dissolved in water they can both accept and donate H+ ions affecting the pH of the solution.
The R group of an amino acid can also accept or donate electrons depending on the functional groups in it. As a result, different amino acids have different isoelectric points.
The charge on an amino acid during electrophoresis depends on how its isoelectric point compares to the pH of the buffer solution used:
• Higher – Those amino acid whose isoelectric point is higher than the pH of the buffer solution, will become positively charged.
• Lower – Those amino acid whose isoelectric point is lower than the pH of the buffer solution, will become negatively charged.
• Equal – Those amino acid whose isoelectric point is equal to the pH of the buffer solution, will remain uncharged.





Still got a question? Leave a comment
Leave a comment