Organic Synthesis - Practical Purification Techniques (A-Level Chemistry)
Practical Purification Techniques
Products of organic reactions may not always be pure. The presence of impurities can affect the reactivity and properties of the products and hence should be removed.
Solvent Extraction
Solvent extraction is a purification technique used when both a compound and its impurities are soluble in water but have different solubilities in an organic solvent.
The organic solvent is chosen so that the desired compound dissolves better in it than in water.
1. Add the impure compound to a separating funnel. An aqueous solution of the impure compound gets added a separating funnel.
2. Add some organic solvent. Shake well. The product will dissolve in the organic compound and the impurities will be left dissolved in the water.
3. Two layers form. The organic solvent and water are immiscible so two layers will form in the separating funnel: the top layer containing the product dissolved in the organic solvent, and the bottom layer containing the impurities dissolved in water.
4. Let the layers run off separately. Each layer is run off into different containers. As the impurities are on the bottom layer, they will get run off first.
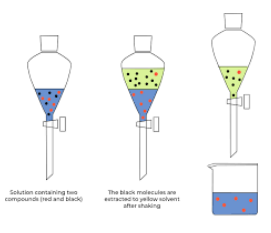
Solvent extraction is not 100% efficient as some of the product will remain dissolved in the water.
Filtration
Filtration is used to separate a liquid and an insoluble solid.
Depending on whether the desired product is the solid or the liquid (the filtrate), different techniques are used.
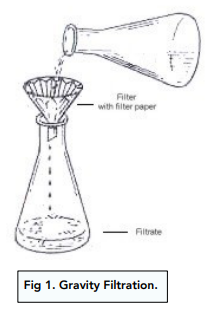
Gravity Filtration
Gravity filtration is used to keep the filtrate.
A piece of flutter filter paper is placed on a funnel, which then gets fitted into a conical flask.
The mixture is poured gently into the filter paper. The solid will remain on top whilst the liquid is able to pass through and collect into the flask.
Filtration Under Reduced Pressure
If you want to keep the solid, filtration under reduced pressure is used instead.
The set up for this separation technique is shown in the figure below.
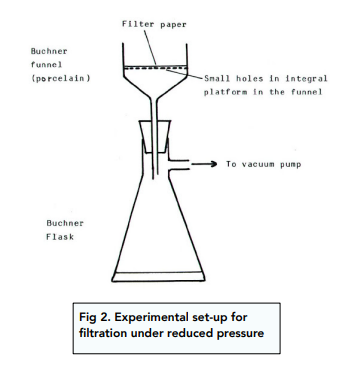
The vacuum gets turned on and the mixture is poured into the Büchner funnel.
As a result of the reduced pressure inside the flask, the liquid gets sucked out of the mixture and down the funnel into the flask. The solid is left behind on the filter paper.
Recrystallisation
Recrystallisation is used to purify solid organic products.
1. Add hot solvent. Add just enough solvent to produce a saturated solution of the impure compound.
2. Filter the hot solution. Remove insoluble impurities by using gravity filtration.
3. Let the solution cool. As the solution cools down, the solubility of the compound decreases.
4. Crystals form. As the solubility of the compound decreases, crystals start to form. As the concentration of impurities is much lower, they take longer to from crystals and hence will remain in solution.
5. Remove the crystals. The crystals are removed by using filtration under reduced pressure and then rinsed with cold solvent. Finally they are let dry.
Solvent choice is very important in recrystallization.
The solubility of the compound in the solvent has to be very high when hot but very low when cold.
If solubility in the cold solvent is too high, most of the compound will not crystallise, remaining in solution instead and getting filtered off.
Distillation
Distillation can be used to purify an organic liquid when the organic compound and its impurities have different boiling points.
When a sample of the impure compound gets heated, the compound and its impurities will boil and get distilled at different temperatures.
Steam Distillation
Organic liquids that have very high boiling points or decompose upon heating cannot be purified using simple distillation.
If these compounds are do not mix with water, steam distillation can be used instead.
Water gets heated and the resulting water vapor is passed into a flask containing a sample of the impure compound.
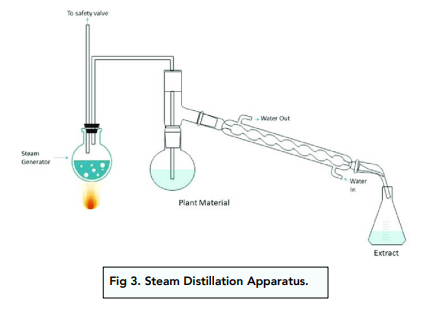
The presence of steam lowers the boiling point of the compound.
As a result, the compound will evaporate at a lower temperature and get distilled before decomposing.
The organic compound and the steam will pass through the condenser together . They can then be separated with a separating funnel.
Purity Indicators
Pure compounds will have defined boiling and melting points.
The greater the amount of impurities present, the more the boiling and melting points of a compound will deviate from those of its pure form.
Boiling Point
The boiling point of an impure compound will be higher than the boiling point of the compound in its pure form.
The boiling points of an organic liquid can be measured using a distillation apparatus.
A sample of the compound gets heated and the temperature at which it just gets distilled will be its boiling point.
This can then be compared the boiling point of the pure compound found in data booklets.
Melting Point
The melting point of an impure compound will be lower than the melting point of the compound in its pure form.
Moreover, its melting range will be broader.
The melting point of an organic solid can be measured using a melting point apparatus.
A capillary tube gets packed with a small amount of solid sample and put into the heating element.
Temperature gets then increased until the solid melts completely.
The melting point of the sample can then be compared to that of the pure compound in data books.
FAQs
In A-Level Chemistry, some common purification techniques include:
Recrystallization: Dissolving a solid impure compound in a hot solvent and then allowing the solution to cool slowly, allowing pure crystals to form.
Fractional distillation: Separating liquids with different boiling points by using a fractionating column, which provides a large surface area for repeated vaporization and condensation of the liquid mixture.
Simple distillation: Separating a liquid from a solid or another liquid by heating the mixture and condensing the vapor to obtain the purified liquid.
Paper chromatography: Separating components of a mixture based on differences in their solubility in a mobile phase and their affinity for a stationary phase, using filter paper as the stationary phase.
Thin-layer chromatography (TLC): Separating components of a mixture based on differences in their solubility in a mobile phase and their affinity for a stationary phase, using a thin layer of silica gel or alumina as the stationary phase.
Gas chromatography (GC): Separating components of a gaseous mixture based on differences in their boiling points or vapor pressures, using a stationary phase coated on a column.
High-performance liquid chromatography (HPLC): Separating components of a liquid mixture based on differences in their solubility in a mobile phase and their affinity for a stationary phase, using a high-pressure pump to move the mobile phase through a column.
Ion exchange chromatography: Separating components based on differences in charge, using a stationary phase that contains charged groups to attract or repel charged molecules.
Gel filtration chromatography: Separating components based on differences in size, using a stationary phase consisting of porous beads that allow smaller molecules to enter the beads and take longer to pass through the column.
These techniques can be used to purify a wide variety of compounds and mixtures, depending on their specific characteristics and the desired level of purity.
Organic synthesis is the process of combining simple organic compounds to form a more complex molecule.
Purification in organic synthesis is the process of removing impurities to obtain a purer product. This helps to improve the quality and yield of the final product.
Common impurities found in organic synthesis include solvents, starting materials, side products, and degradation products.
There are many different techniques for purifying substances, including:
Distillation: Separates components based on differences in boiling points.
Filtration: Separates solids from liquids or gases based on differences in particle size or solubility.
Chromatography: Separates components based on differences in polarity, charge, or size.
Crystallization: Separates components based on differences in solubility at different temperatures.
Extraction: Separates components based on differences in solubility in different solvents.
Fractional crystallization: Separates components based on differences in solubility at different temperatures, followed by repeated crystallization to isolate pure components.
Electrophoresis: Separates components based on differences in charge and size.
Ion exchange: Separates components based on differences in charge.
Ultrafiltration: Separates components based on differences in size and shape.
Precipitation: Separates components based on differences in solubility.
These techniques can be used alone or in combination to achieve a high degree of purity for a given substance. The choice of purification technique depends on the specific characteristics of the substance being purified, as well as the desired level of purity and the amount of material to be purified.
Distillation works by heating a mixture of organic compounds to a temperature where one component will vaporize. The vapor is then condensed and collected, resulting in a purified product.
Crystallization is a technique used to purify solid compounds. The compound is dissolved in a solvent and then allowed to cool and form crystals. The crystals are then separated from the solvent to obtain a pure product.
Recrystallization is a type of crystallization in which the compound is dissolved in a hot solvent, then allowed to cool and form crystals. The difference is that the solvent used in recrystallization is carefully chosen to dissolve impurities and leave the desired compound in its pure form.
Filtration is a process in which a mixture is passed through a filter to separate solid impurities from the desired liquid product. Filtration is often used in organic synthesis to remove solid impurities from a reaction mixture.
Chromatography is a technique used to separate mixtures of compounds based on their physical and chemical properties. It is often used in organic synthesis to purify complex mixtures of compounds.






Still got a question? Leave a comment
Leave a comment