Transition Metals - Complex Ion Shape (A-Level Chemistry)
Complex Ion Shape
Shapes of Complexes
Like all covalent molecules, transition metal complexes have shapes due to their co-ordinate covalent bonding with ligands.
Co-ordination Number: 6
If the transition metal complex has a coordination number of 6, with small ligands like water or ammonia, the shape of the complex is octahedral with a bond angle of 90°.
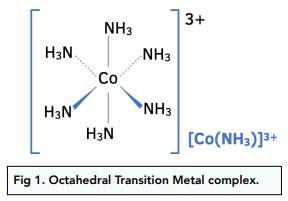
Likewise, bidentate and multidentate ligands with a co-ordination number of 6, will also have an octahedral shape.
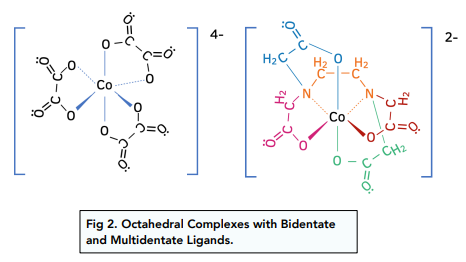
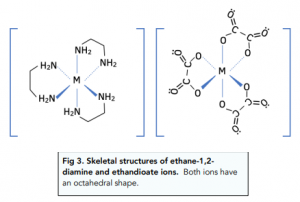
Co-ordination Number: 4
If the transition metal complex has larger ligands, like chloride ions, it has a co-ordination number of 4. Fewer larger ligands can fit around the central transition metal. The shape is either tetrahedral or square planar.
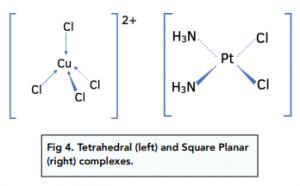
The tetrahedral shape [Cu(Cl)₄]²- has a bond angle of 109.5°.
The square planar shape [Pt(NH₃)₂(Cl)₂] has a bond angle of 90°.
Co-ordination Number: 2
If the transition metal complex has a co-ordination number of two, the shape is linear. The bond angle is 180°.
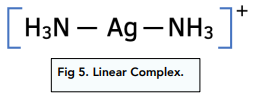
This linear complex silver diammine (I) [Ag(NH₃)₂]⁺ is used in Tollen’s reagent, which is used to test for the presence of aldehydes.
If aldehydes are present, a silver mirror forms around the tube, as the Ag⁺ ions are reduced to Ag metal.





Still got a question? Leave a comment
Leave a comment