Thermodynamic - Introduction to Entropy (A-Level Chemistry)
Introduction to Entropy
Entropy
Key Terms
Entropy can be defined as the randomness or dispersal of energy of a system. The more disordered a system is, the higher (the more positive) the value of entropy.
Entropy is affected by:
1. State of Matter – Generally, solids have lower entropy than liquids, which have lower entropy than gases. This is because the arrangement of particles is more random in gases than solids.
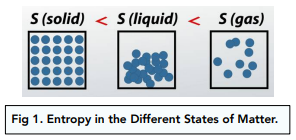
2. Dissolving – When a solid dissolves in a solvent, its entropy increases because of the free movement of the dissolved particles.
3. Number of particles – For an increased number of particles there is and increased number of ways in which these can be arranged and therefore an increased entropy. When in a reaction the number of moles of product formed is greater than the number of moles of reactants, there is an increase in entropy.
The more disordered a system is, the higher its entropy and the more stable it is. Therefore, substance will naturally move in order to increase their entropy.
Entropy Changes
In a chemical reactions, products and reactants have different entropies – there is an entropy change.
For reactions with a negative entropy change, reactants will have a higher entropy than products.
For reactions with a positive entropy change, products will have a higher entropy than reactants.
Calculating Entropy Changes
Entropy values have been determined and can be found in databases. They are typically found at standard conditions.
The formula for calculating ΔS is as follows:
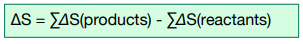
This is just the difference in entropy between the sum of the products and the sum of the reactants.
Example: Calculate the entropy change for this reaction:
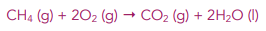

1. Calculate the entropy of the products. You can work out the entropy of the products by adding up the entropy values for each product in a reaction
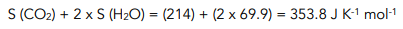
2. Calculate the entropy of the reactants. You can work out the entropy of the reactants by adding up the entropy values for each reactant in a reaction
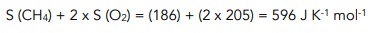
3. Calculate the entropy change for the reaction using the equation. Notice the negative sign, this indicates that entropy has decreased in the reaction. This is as we would expect since we have gone from two gases to a gas and a liquid. A liquid is more ordered than a gas, therefore entropy has decreased.
ΔS =∑Δ S (products) – ∑ΔS (reactants)
ΔS = 353.8 – 596 = -242.2 J K-1 mol-1
Thermodynamics is the branch of science that deals with the relationships between heat, energy, and work. Entropy is a fundamental concept in thermodynamics, which refers to the measure of the degree of disorder or randomness in a system.
The second law of thermodynamics states that the total entropy of an isolated system always increases over time. This means that in any process, the overall disorder or randomness of the system will increase.
The entropy of a system is related to its energy and temperature, as stated by the equation ΔS = ΔQ/T, where ΔS is the change in entropy, ΔQ is the heat added to the system, and T is the absolute temperature.
Reversible processes are idealized processes that can be reversed with no change in entropy, while irreversible processes always result in an increase in entropy. In a reversible process, the entropy change is equal to zero, while in an irreversible process, the entropy change is greater than zero.
Gibbs free energy is a thermodynamic quantity that relates the enthalpy, entropy, and temperature of a system. The Gibbs free energy is related to the entropy by the equation ΔG = ΔH – TΔS, where ΔG is the change in free energy, ΔH is the change in enthalpy, and TΔS is the change in entropy. The sign of ΔG determines whether a process is spontaneous or not.
The change in entropy of a system is related to the direction of a chemical reaction. A positive change in entropy (ΔS > 0) indicates that the reaction is spontaneous in the forward direction, while a negative change in entropy (ΔS < 0) indicates that the reaction is spontaneous in the reverse direction.
The study of entropy has many practical applications, including the design of efficient engines, the prediction of phase transitions in materials, the development of new materials, and the study of biological systems. The concept of entropy is also important in environmental science, where it is used to study the impact of human activity on the natural world.





Still got a question? Leave a comment
Leave a comment