Properties of Period 3 Elements - Properties of Period 3 Compounds (A-Level Chemistry)
Properties of Period 3 Compounds
Period 3 Oxides
We can observe trends going across period 3, we can use the oxides of these elements to see these trends.
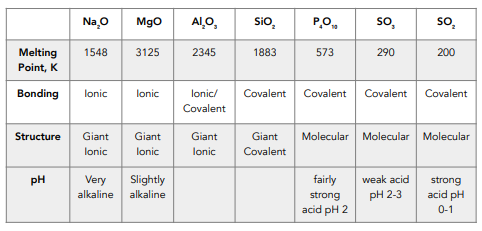
Melting Points of Oxides
Melting points depend on the strength of the forces of attraction between atoms, ions or molecules.
The strength of the forces in ionic lattices and giant covalent structures are higher than molecules with simple covalent bonding.

The melting points of sodium oxide, magnesium oxide and aluminium oxide are particularly higher than the other period 3 oxides because they are giant ionic lattice structures. This is where the bonding extends throughout the entire structure so lots of energy is required to overcome these forces.
The melting point of aluminium oxide is lower than might be expected due to the covalent character it shows. The highly charged, highly polarising Al³⁺ ion distorts the electron cloud of the O²⁻ ion, adding some covalent character.

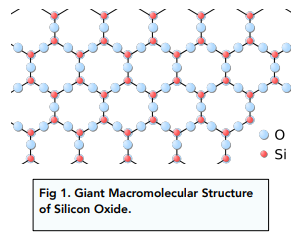
Silicon dioxide has a noticeably higher melting point than the other nonmetal oxides because of its giant macromolecular structure. Its covalent bonding extends throughout the entire macromolecule. Large amounts of energy are required to overcome those strong covalent bonds.

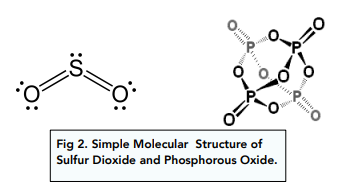
Phosphorus oxide and sulfur dioxide have relatively low melting points because of their structures. They both form simple molecular structures so only have weak intermolecular forces that need to be overcome to
melt them.
The larger the covalent molecule, the greater the Van der Waals forces, so more energy is required to separate molecules and the melting points are higher. This is why P4O10 has a higher melting point compared to SO2.
Reactions of Oxides with Water
Basic Oxides + Water
Sodium and magnesium both form oxides that are basic.
• Sodium oxide – will react with water to form a highly alkaline solution of sodium hydroxide:
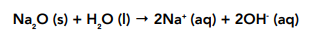
Sodium oxide contains the strongly basic O²⁻ ion, this can strongly attract protons therefore it will readily react with water to form a strongly alkaline solution containing hydroxide ions as shown above.
• Magnesium oxide – will react with water to form a less alkaline solution than sodium oxide formed. It forms a solution of magnesium hydroxide with a pH of around 9. Magnesium hydroxide is sparingly soluble in water.
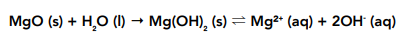
Magnesium oxide is less soluble than sodium oxide, therefore the magnesium hydroxide produced by the reaction of magnesium oxide with water will only partially dissociate into its ions, a less alkaline solution than with sodium oxide is formed.
Insoluble Oxides
Aluminium oxide and silicon dioxide are both insoluble in water.
• Aluminium oxide – The bonding within the structure of aluminium oxide is too strong to be broken, the ions cannot be separated.
• Silicon dioxide – The giant macromolecular structure of silicon dioxide makes it insoluble in water.
Acidic Oxides + Water
The non-metal oxides, phosphorus oxide and the sulfur oxides, are typically acidic.
• Phosphorus pentoxide – will react fairly violently with water, forming a solution with a pH of between 0 and 2. The phosphoric (V) acid produced will ionise, to give an acidic solution.
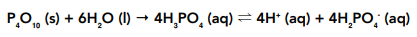
Phosphoric acid does not dissociate completely. It has a pH of about 2.
• Sulfur dioxide – is quite soluble in water and will also react with water giving a weakly acidic solution of partially dissociating sulfuric (IV) acid.
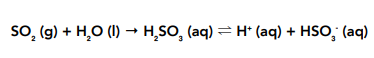
• Sulfur trioxide – will also react with water, quite violently, to form a solution of sulfuric (VI) acid. This dissociates completely forming a strong acid.
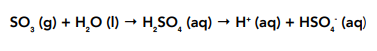
Reactions With Acids and Bases
Sodium and Magnesium Oxides + Acid
Both sodium oxide and magnesium oxide will react with an acid to produce a salt and water.
e.g. Sodium oxide:
Na₂O (s) + 2HCl (aq) → 2NaCl (aq) + H₂O (l)
e.g. Magnesium oxide:
MgO (s) + H₂SO₄ (aq) → MgSO₄ (aq) + H₂O (l)
Sodium and magnesium hydroxides behave as alkalis. They react with acids to produce a metal salt and water in a typical neutralisation reaction.
Aluminium Oxide + Acid / Bases
Aluminium oxide is an amphoteric oxide. This means it can react with both an acid or an alkali.
e.g. reaction with an acid:
Al₂O₃ (s) + 6HCl (aq) → 2AlCl₃ (aq) + 3H₂O (l)
e.g. reaction with a base:
Al₂O₃ (s) + 2NaOH (aq) + 3H₂O (l) → 2NaAl(OH)₄ (aq)
The product is called sodium aluminate.
Aluminium hydroxide also shows amphoterism and will react with both acids and bases to form a salt and water.
Silicon Dioxide + Bases
Silicon dioxide can react as a weak acid with strong bases:
SiO₂ (s) + 2NaOH (aq) → Na₂SiO₃ (aq) + H₂O (l)
The product is called sodium silicate.
Phosphorus Pentoxide + Bases
When phosphorus pentoxide reacts with water, phosphoric (V) acid is produced.
Therefore the reaction that occurs between phosphorus pentoxide and a base is actually a reaction between phosphoric (V) acid and a base.
Three moles of sodium hydroxide are needed to completely react with phosphoric (V) acid, this is because it has three H+ions which can dissociate:
H₃PO₄ (aq) + NaOH (aq) → NaH₂PO₄ (aq) + H₂O (l)
NaH₂PO₄ (aq) + NaOH (aq) → Na₂HPO₄ (aq) + H₂O (l)
Na₂HPO₄ (aq) + NaOH (aq) → Na₃PO₄ (aq) + H₂O (l)
We can simplify these three stages by summarising them all in one overall equation:
3NaOH (aq) + H₃PO₄ (aq) → Na₃PO₄ (aq) + 3H₂O (l)
Sulfur Dioxide + Bases
Sulfur dioxide will react with a base to first form sodium hydrogensulfate (IV):
SO₂ (aq) + NaOH (aq) → NaHSO₃ (aq)
This sodium hydrogen (IV) sulfate will then go on to react with another sodium hydroxide to form sodium (IV) sulfate:
NaHSO₃ (aq) + NaOH (aq) → Na₂SO₃ (aq) + H₂O (l)
Sulfur Trioxide + Bases
Sulfur dioxide will react with readily with bases:
SO3 (aq) + 2NaOH (aq) → Na2SO4 (aq) + H2O(l)
Period 3 Chlorides
All Period 3 elements can form chlorides when they react with chlorine. These compounds show a characteristic behaviour when added to water.
Metal Chlorides + Water
Sodium chloride and magnesium chloride are ionic compounds. When added to water, ion-dipole interactions form between the polar water molecules and the ions in the lattice so that the ionic lattice dissociates. The positively charged metal ions and the negatively charged chloride ions become surrounded by water molecules to form
hydrated ions.
e.g. Sodium chloride
NaCl (s) → Na+ (aq) + Cl- (aq)
e.g. Magnesium chloride
MgCl2 (s) → Mg+2 (aq) + 2Cl- (aq)
The resulting solutions has a neutral pH of 7.
Aluminium Chloride + Water
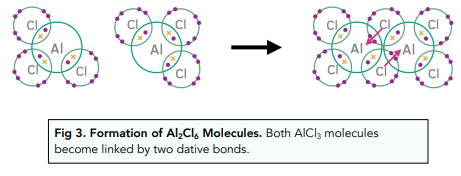
In solid hydrated crystals, aluminium chloride exists as an ionic lattice with formula AlCl3.
However, when water is removed, aluminium oxide exists as a dimer of two AlCl3 molecules covalently bonded together with formula Al2Cl6.By adding water, the dimer breaks apart and aluminium and chloride ions enter into solution.
Aluminium ions have a very high charge density so that it forms very strong ion-dipole interactions with water molecules. These interactions are so strong that it weakens O-H bonds in water molecules causing them to lose a proton, leaving a OH- ion still attached to the Al+³ ion.
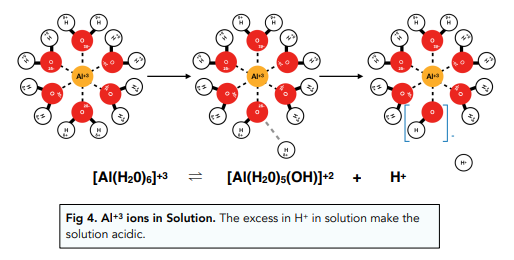
The increase in H+ in solution causes its pH to be slightly acidic (around a pH of 3).
Non-Metal Chlorides + Water
When added to water, silicon chloride and phosphorous (V) chloride rapidly undergo hydrolysis giving off white HCl fumes.
e.g. Silicon chloride
SiCl4 (l) + 2H2O (l) → SiO2 (s) + 4HCl (g)
SiO2 is insoluble and is seen as a white precipitate. Some of the gaseous HCl dissolves in excess water to form an acidic solution (pH = 2).
e.g. Phosphorous (V) chloride
PCl5 (s) + 4H2O (l) → H3PO4 (aq) + 5HCl (g)
Both products can dissolve in excess water so that the solution is very acidic.
Period 3 elements are the elements in the third row of the periodic table, including sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar). These elements have unique chemical and physical properties, including:
High reactivity: Some period 3 elements, such as sodium and magnesium, are highly reactive and readily form compounds with other elements.
Alkali and Alkaline Earth Metals: Sodium and magnesium are classified as alkali metals, which are known for their high reactivity and low ionization energies.
Diatomic molecules: Chlorine, for example, is a gas that exists as diatomic molecules (Cl2).
Non-metal Characteristics: Silicon and phosphorus are metalloids, which means they have properties of both metals and non-metals. Sulfur and chlorine are non-metals.
Period 3 compounds are the compounds formed by the combination of period 3 elements. The properties of these compounds are determined by the properties of the elements they contain and their chemical bonds. Some common properties of period 3 compounds include:
Ionic Compounds: Compounds such as sodium chloride (NaCl) are ionic, meaning they contain ions and are held together by ionic bonds. These compounds typically have high melting and boiling points.
Covalent Compounds: Compounds such as silicon dioxide (SiO2) and sulfur dioxide (SO2) are covalent, meaning they contain covalent bonds between atoms. These compounds typically have low melting and boiling points.
Acids: Compounds such as sulfuric acid (H2SO4) and phosphoric acid (H3PO4) are acids, meaning they can donate hydrogen ions (protons) to a solution.
Bases: Compounds such as sodium hydroxide (NaOH) and magnesium hydroxide (Mg(OH)2) are bases, meaning they can accept hydrogen ions and neutralize acids.
A-Level Chemistry is an advanced level of secondary education in many countries, and period 3 elements and compounds play an important role in this curriculum. Understanding the properties of these elements and compounds is essential for understanding many fundamental chemical concepts, including:
The reactivity of elements and the formation of compounds
The nature of ionic and covalent bonds
The properties of acids and bases
The properties and behavior of solids, liquids, and gases
In A-Level Chemistry, students are expected to understand the properties of period 3 elements and compounds, and to use this knowledge to predict chemical reactions and explain chemical phenomena.





Still got a question? Leave a comment
Leave a comment