Bonding - Forces Between Molecules (A-Level Chemistry)
Forces Between Molecules
Intermolecular Forces
Types of Intermolecular Forces
An intermolecular force is the force that arises from the interaction between molecules. Intermolecular attractions are not nearly as strong as the intramolecular attractions that hold compounds together (covalent or ionic bonds).
There are three main types of intermolecular force:
- Permanent dipole-dipole forces
- Induced dipole-dipole forces – Also known as van der Waals, dispersion or London forces.
- Hydrogen bonding
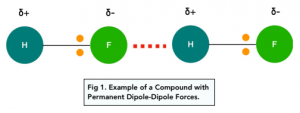
1. Permanent Dipole-Dipole Forces
Dipoles in Polar Molecules
Permanent dipole-dipole forces are the weak intermolecular forces of attraction that arise between permanently polar molecules. These forces are between the delta positive end of one polar bond with the delta negative end of another polar bond.
The more polar the molecules, the stronger the force of attraction between them.
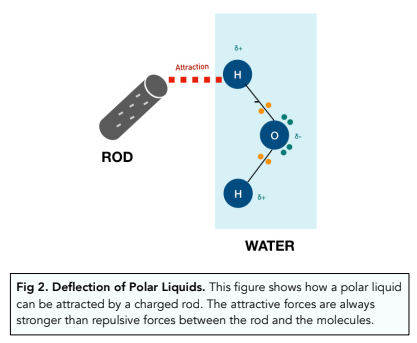
Charged Rod Experiment
If you charge a rod with a cloth by rubbing it and then place it next to water, the liquid will be attracted to the rod. This is because water is a polar liquid made up of polar molecules. The charges on the rod will attract the oppositely charged ions in water. For example if the rod is charged with negative charge, it will attract the delta positive charges of water.
2. Induced Dipole-Dipole Forces
Formation of Temporary Dipoles
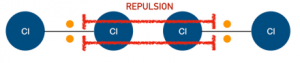
Induced dipole-dipole forces are weak intermolecular forces of attraction present between all atoms and all molecules that exist – whether polar or non-polar – as a result of the present of electrons in the molecule and the formation of temporary dipoles. They are the weakest type of intermolecular force.
When two atoms comes towards each other, the electron clouds of these atoms repel each other.
This causes a sudden displacement of electrons in one atom to one side causing the atom to have a temporary dipole: a transient asymmetry of charge distribution around an atom.
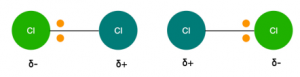
Once a temporary dipole is formed, it induces temporary dipoles in neighbouring atoms and molecules in a domino like effect. The two temporary dipoles are attracted towards each other by induced dipole- dipole forces also known as London or Van der Waals forces.
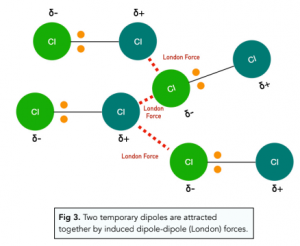
The dipoles are called temporary because as electrons are constantly moving, dipoles are constantly being created and destroyed. However, there is always a dipole present at any given time, so overall effect is for the atoms in the molecule to remain attracted to each other.
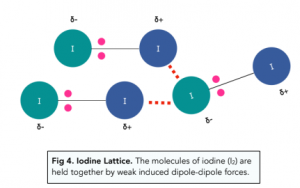
Example: Iodine Lattice
A lattice made of molecules is held together by induced dipole-dipole forces.
For example lots of iodine molecules can form a lattice at room temperature when iodine exists in the solid state. The molecules of iodine (I2) are held together by weak induced dipole-dipole forces in a lattice structure even though the iodine molecule itself consists of a strong covalent bond.
3. Hydrogen Bonding
Formation of Hydrogen Bonds
Hydrogen bonds are a special type of permanent dipole-dipole forces that only form when hydrogen forms a covalent bond with a very electronegative element: either nitrogen, oxygen or fluorine. They are the strongest type of intermolecular force.
As nitrogen, oxygen and fluorine are more electronegative hydrogen, they attract the electron pair in the covalent bond with hydrogen towards themselves. This forms a polar bond – hydrogen is δ+, and the other atom is δ-.
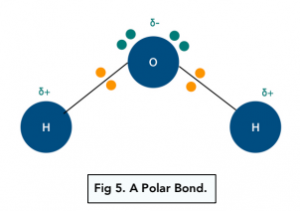
Hydrogen atoms have a very high charge density because the hydrogen atoms only have 1 shell of electrons. Therefore the δ+ hydrogen can be attracted to the lone pair of electrons on the nitrogen, oxygen or fluorine atoms in nearby molecules.
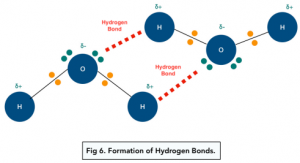
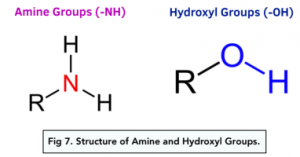
Amine and Hydroxyl Groups
The presence of amine groups (-NH) or hydroxyl groups (-OH) indicates hydrogen bonding.
Both of these groups contain one of the more electronegative elements: nitrogen, oxygen and fluorine as well as a hydrogen atom. For example, water and ammonia both contain these groups and both consist of hydrogen bonding.
Properties and Different Types of Intermolecular Forces
Strength of Intermolecular Forces
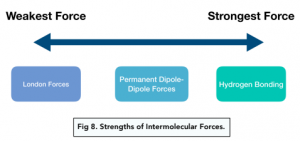
London forces are the weakest force, and are found in nearly all molecules. Non-polar molecules only have London forces.
Hydrogen bonds are the strongest force.
Permanent dipole-dipole forces are stronger than London forces, but weaker than hydrogen bonds.
Intermolecular Forces and Physical Properties
The types of intermolecular forces that form between molecules of a compound will contribute to determining many of the compound’s physical properties such as melting or boiling point.
The stronger the intermolecular forces, the more energy needed to break them apart and hence the higher the melting and boiling points of the compound.
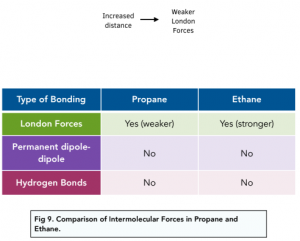
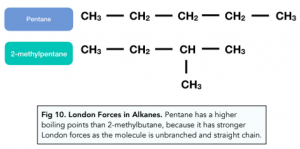
London Forces in Alkanes
The boiling point of some molecules is determined by the strength of the intermolecular forces. These often need to be broken upon boiling or melting.
The strength of London forces is determined by three factors:
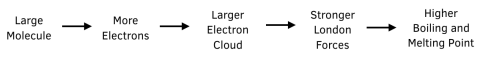
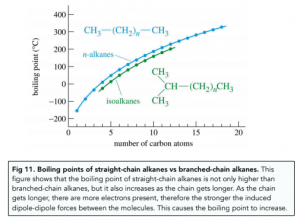
- Molecular size – a larger molecule contains more electrons, therefore it consists of larger electron clouds. The greater the number of electron clouds, the stronger the induced dipole-dipole forces. More energy is required to break stronger induced dipole-dipole forces, therefore the boiling and melting point is higher.
- Molecular shape – a molecule with a greater surface area consists of stronger induced dipole-dipole forces. For example, long-chain and straight-chain alkanes have larger molecular surface areas than short-chain and branched-chain alkanes.

- Distance between molecules – if molecules can pack together closely, then they will have stronger intermolecular forces between them. For example, unbranched, straight-chain alkanes can pack together better than branched chain alkanes.
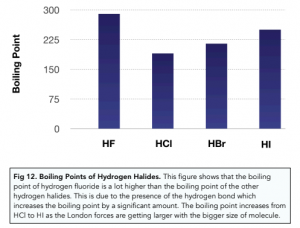
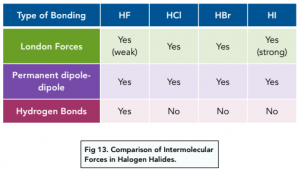
Hydrogen Bonds in Hydrogen Halides
As hydrogen bonds are the strongest type of intermolecular forces, the greatest amount of energy is needed to overcome these forces. This means that the melting and boiling points of compounds which can form hydrogen bonds are higher.
FAQs
Intermolecular forces are the attractive or repulsive forces that exist between molecules in a substance. These forces are weaker than chemical bonds that hold atoms together within molecules, but they play an important role in determining the physical properties of a substance, such as its melting and boiling points, viscosity, and solubility.
Understanding intermolecular forces is important in chemistry, as it helps us to predict the physical properties of substances and their behavior under different conditions. For example, knowledge of intermolecular forces is important in designing solvents for chemical reactions and in understanding the properties of materials such as polymers, plastics, and pharmaceuticals.
The three types of intermolecular forces in A-level chemistry are:
London dispersion forces: These are the weakest type of intermolecular force and exist between all molecules, regardless of their polarity. London dispersion forces arise due to temporary dipoles that are formed when electrons in a molecule are unevenly distributed.
Dipole-dipole forces: These are stronger than London dispersion forces and exist between polar molecules. Dipole-dipole forces arise due to the attraction between the positive end of one molecule and the negative end of another molecule.
Hydrogen bonding: This is the strongest type of intermolecular force and exists between molecules that contain hydrogen bonded to highly electronegative elements such as nitrogen, oxygen, or fluorine. Hydrogen bonding arises due to the attraction between a hydrogen atom and a lone pair of electrons on an electronegative atom in a nearby molecule.
Understanding the different types of intermolecular forces is important in predicting the properties of molecules, such as their boiling points, melting points, and solubility. It is also important in understanding the behavior of molecules in different environments, such as in solution or in the gas phase.
Bonding forces are the attractive forces between atoms or molecules that hold them together.
The different types of bonding forces are ionic bonding, covalent bonding, hydrogen bonding, and van der Waals forces.
Ionic bonding is the type of bonding that occurs between ions of opposite charge. It is formed by the transfer of electrons from one atom to another.
Covalent bonding is the type of bonding that occurs between atoms that share electrons. It results in the formation of a covalent bond between the atoms.
Hydrogen bonding is a type of intermolecular force that occurs between molecules containing hydrogen atoms that are covalently bonded to highly electronegative atoms such as nitrogen or oxygen.
Intermolecular forces are the forces of attraction or repulsion that exist between molecules in a substance. These forces arise due to the interactions between the electrically charged particles (electrons and nuclei) that make up the molecules.
The properties of intermolecular forces include:
Strength: Intermolecular forces vary in strength, with London dispersion forces being the weakest and hydrogen bonding being the strongest.
Effect on physical properties: Intermolecular forces determine the physical properties of a substance, such as its melting and boiling points, viscosity, and surface tension. For example, substances with strong intermolecular forces tend to have higher melting and boiling points than substances with weak intermolecular forces.
Solubility: Intermolecular forces also affect the solubility of a substance in a solvent. Substances with similar intermolecular forces tend to be more soluble in each other, while substances with different intermolecular forces tend to be less soluble.
Effect on chemical reactions: Intermolecular forces can also affect the rates and outcomes of chemical reactions. For example, a polar solvent can stabilize the charged intermediates formed during a reaction and increase the rate of the reaction.
Understanding the properties of intermolecular forces is important in many areas of chemistry, including materials science, drug design, and biochemistry.
Attractive forces between molecules are intermolecular forces that arise due to the interactions between the electrically charged particles (electrons and nuclei) that make up the molecules. These forces are responsible for the cohesion of liquids and solids, and they determine many of the physical and chemical properties of substances.
There are several types of attractive forces between molecules, including:
London dispersion forces: These are the weakest type of attractive force and arise due to temporary dipoles that are formed when electrons in a molecule are unevenly distributed.
Dipole-dipole forces: These are stronger than London dispersion forces and exist between polar molecules. Dipole-dipole forces arise due to the attraction between the positive end of one molecule and the negative end of another molecule.
Hydrogen bonding: This is a special type of dipole-dipole force that exists between molecules that contain hydrogen bonded to highly electronegative elements such as nitrogen, oxygen, or fluorine. Hydrogen bonding is particularly strong and contributes to the high boiling points of substances such as water and ammonia.
Ion-dipole forces: These are the strongest type of attractive force and exist between an ion and a polar molecule. Ion-dipole forces play an important role in many chemical reactions and in the behavior of ions in solution.
Understanding attractive forces between molecules is important in many areas of chemistry, including materials science, drug design, and biochemistry.
Understanding bonding forces is important in A-Level Chemistry as it helps explain the properties of different substances and how they interact with each other. It is also a fundamental concept for understanding chemical reactions and the behaviour of molecules.






Still got a question? Leave a comment
Leave a comment