Amount of Substance - Stoichiometric Calculations (A-Level Chemistry)
Stoichiometric Calculations
Stoichiometry
Definitions
Stoichiometric coefficients are the numbers that precede a compound in a balanced chemical reaction. They tell us about the relative proportions of reagents and products in a reaction or in other words, give us the mole ratio of reactants to products.
For example, in the balanced equation re the numbers that recede a compound in a balanced chemical reaction Fe2O3 + 2Al —> 2Fe + Al2O3 , the stoichiometric coefficients are given in blue and the mole ratio is 1:2:2:1.
Stoichiometry is the use of balanced equations to calculate amounts of reagents and products.
Calculating Mass from a Balanced Equation
You can use balanced equations to work out the masses of substances that form or react in reactions.
Practice Question: What mass of magnesium oxide is formed when 12g of magnesium is completely combusted in air? (Ar values are as follows, Mg = 24.3, O = 16)
1. First of all write out a balanced equation for the reaction.
2Mg(s) + O2(g) → 2MgO(s)
2. Next write down the relative molecular mass (Mr) or relative atomic mass (Ar) of the substances mentioned in the question.
In this case the substances mentioned are magnesium oxide and magnesium.
Ar of Magnesium (Mg) = 24.3
Mr of Magnesium Oxide (MgO) = 24.3 + 16.0 = 40.3
3. Then calculate the number of moles of magnesium that reacts in the equation.

Number of moles of Mg that reacts = 12g ÷ 24.3 = 0.494 moles
So approximately 0.5 moles of Mg reacts.
4. Now calculate how many moles of magnesium oxide are formed
We can calculate the number of moles of MgO made from the number of moles of Mg we calculated in step 3. We find this by comparing the coefficient number on either side of the equation.
The coefficient numbers show that 2 moles of Mg make 2 moles of MgO. This means the the ratio of Magnesium to Magnesium oxide in the reaction is 2:2 = 1:1
This means that if 0.5 moles of Magnesium react in this reaction, then 0.5 moles of Magnesium Oxide is formed in the reaction.
5. Finally calculate the mass of Magnesium oxide formed

Mass of MgO formed = number of moles x Mr
Mass of MgO formed = 0.5 x 40.3 = 20.15g
Calculating Volume from a Balanced Equation
You can also use balanced equations to work out the volume of gases that form or react in reactions.
Practice Question: Calculate the volume of gas in m3 formed when 35g of magnesium reacts with excess water. The reaction takes place at a temperature of 268K and a pressure of 156000 Pa. The gas constant is 8.31 J K-1mol-1. (Ar values are as follows, Mg = 24.3, O = 16)
1. First of all write out a balanced equation for the reaction.
Mg (s) + 2H2O (l) → Mg(OH)2 (s) + H2 (g)
2. Next write down the relative molecular mass (Mr) or relative atomic mass (Ar) of the substances mentioned in the question.
In this case the substance mentioned is magnesium (Mg).
Ar of Magnesium (Mg) = 24.3
3. Then calculate the number of moles of magnesium that reacts in the equation.

Number of moles of Mg that reacts = 35g ÷ 24.3 = 1.44 moles (to 3 significant figures)
4. Now calculate how many moles of hydrogen are formed.
From the equation, we can see that the the ratio of Magnesium to Hydrogen in the reaction is 1:1
This means that if 1.44 moles of Magnesium react in this reaction, then 1.44 moles of Hydrogen gas is formed in the reaction.
5. Finally calculate the volume of Hydrogen gas formed by again using the formula:

Volume of Hydrogen formed = nRT ÷ p = (1.44 x 8.31 x 268) ÷ 156000 = 0.0206 m3
Summary
If in doubt of what calculations to make, refer back to this diagram:
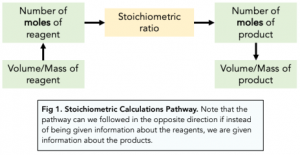
Limiting and Excess Reagents
Stoichiometric ratios tell us about the exact amount of reagents that are needed for the reaction to proceed. If there is not enough of one reactant then the reaction will stop.
The limiting reagent is the reagent that is completely used up by the end of the reaction. It will limit how much of the products are made, since the reaction will not be able to proceed without it even if the are still some of the other reagents left over.
The reagents that are left over after the limiting regent is completely consumed, are the excess reagents.
Practice Question: Aluminum metal reacts with chlorine gas to give aluminum chloride. What is the limiting reagent if we start with 2.80g of Al and 4.25g of Cl2?
1. First of all write out a balanced equation for the reaction.
2Al (s) + 3Cl2 (g) →2AlCl3 (g)
2. Next write down the relative molecular mass (Mr) or relative atomic mass (Ar) of the substances mentioned in the question.
Ar of Aluminum (Al) = 27.0
Mr of Chlorine (Cl2) = 35.5 x 2 = 71
3. Then calculate the number of moles of each reagent that react in the equation.
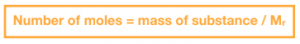
Number of moles of Al that reacts = 2.80g ÷ 27 = 0.104 mol (to 3 significant figures)
Number of moles of Cl2 that reacts = 4.25g ÷ 71= 0.0599 mol (to 3 significant figures)
4. Calculate the molar ratio of the reactants from the given data and compare to the stoichiometric ratio of the balanced reaction.
Actual ratio = Al moles : Cl2 moles
= 0.104 : 0.0599
= 1.74 : 1
For every mole of Cl2 we have 1.74 moles of Al
Stoichiometric ratio = Al moles : Cl2 moles
= 2 : 3
= 0.67 : 1
For every mole of Cl2 we actually just need 0.67 moles of Al
5. Decide which reagent is in excess and which is the limiting reagent.
We have more Al than we need to react with the given amount of Cl2.
Therefore Al is the excess reagent and Cl2 is the limiting reagent.
FAQs
The concept of amount of substance refers to the quantity of a substance that is present in a sample. It is a measure of the number of particles, ions, or atoms in a sample and is used in chemical calculations to determine the ratios of reactants and products in a reaction.
Stoichiometric calculations are important in A-Level Chemistry because they help us to determine the amounts of reactants and products involved in a reaction. These calculations are based on the balanced chemical equation for the reaction, which gives the ratio of the number of moles of each substance involved in the reaction.
Stoichiometric calculations are performed by using the balanced chemical equation for the reaction and the molar masses of the substances involved. The number of moles of each substance is calculated and used to determine the ratio of the reactants and products. From this information, we can calculate the amount of each substance required to produce a specific amount of product.
A mole is a unit of measurement that is used to express the amount of a substance in a sample. It is defined as the amount of a substance that contains the same number of particles as there are in 12 grams of pure carbon-12. The mole allows us to quantify the number of particles, ions, or atoms in a sample, and is used in stoichiometric calculations.
The molar mass of a substance is the mass of one mole of the substance, expressed in grams. It is the sum of the atomic masses of all the atoms in a molecule of the substance. The molar mass is used in stoichiometric calculations to convert the number of moles of a substance to its mass.
The relationship between moles, mass, and number of particles in a substance can be expressed as follows: 1 mole of a substance contains Avogadro’s number of particles, and the mass of 1 mole of a substance can be calculated using its molar mass. This allows us to convert between the number of moles and the mass of a substance, and to calculate the number of particles in a sample.
The purpose of stoichiometric calculations in chemical reactions is to determine the amount of each reactant and product involved in the reaction. This information can be used to calculate the theoretical yield of a reaction, and to optimize the reaction conditions to maximize the yield. Stoichiometric calculations are also useful for predicting the amount of waste produced by a reaction, and for determining the amounts of reactants required to produce a specific amount of product.





Still got a question? Leave a comment
Leave a comment