Carboxylic Acids and Derivatives - Properties and Reactivity of Esters (A-Level Chemistry)
Properties and Reactivity of Esters
Esters
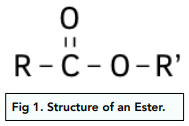
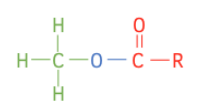
Structure of Esters
Esters are molecules derived from carboxylic acids. The O-H group of the acid is replaced with O-R’ group, where R’ is an alkyl group.
Esters have the functional group RCOOR’. The general formula is:
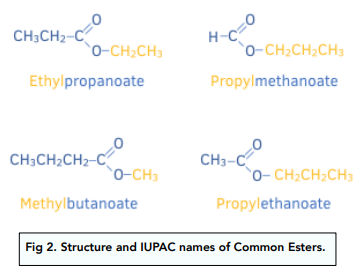
IUPAC Naming of Esters
As esters are derived from carboxylic acids, the name of the ester is based on the parent acid is has come from.
The acid part always forms the suffix of the name, and the prefix is always the alkyl group, R’ in the diagram above, which has replaced the H from the acid.
Some examples are shown below.
Worked Example: Give the IUPAC name for the ester shown
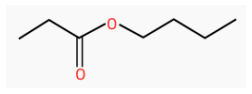
Answer: Butylpropanoate
Explanation: The first part of the molecule is derived from the acid, and has three C atoms. It will therefore form the suffix of the name = propanoate. The part which has replaced the “H” has four carbon atoms, and will form the prefix = butyl.
Common Uses of Esters
Esters have multiple uses, for example:
- Flavours and fragrances – Esters are responsible for many scents in the natural world (e.g. cherry, banana and pear flavours) and many flower fragrances (e.g. lavender are all produced by esters). Ethyl ethanoate smells like pear drops. Esters are made artificially to reproduce these desired flavours and fragrances in foods and perfumes.
- Solvents – Ethyl ethanoate has a low toxicity, volatile and is relatively cheap. This makes it particularly useful as a solvent in glues, medicines and fragrances.
- Plasticisers – A plasticiser is a substance which improves the flexibility of a plastic. PVC is a rigid polymer used in drain pipes. Treating the PVC with a plasticising ester, changes its structure, and enables it to be made into cling film which is used as a food wrapping. The ester molecules penetrate between the polymer chains, increasing the distance between the chains, and so enable the chains to slide over each other. This results in a more flexible plastic.
Hydrolysis of Esters
Esters can be hydrolysed; this means they can be split apart using water.
The reaction is faster in acidic or alkaline conditions.
Hydrolysis in Acidic Conditions
When an ester is heated under reflux with dilute acid (acid hydrolysis), a carboxylic acid and alcohol are formed.
For example:
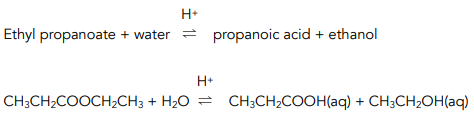
The reaction is reversible and the yield of products is poor.
Hydrolysis in Alkaline Conditions
When an ester is heated under reflux with dilute alkali (base hydrolysis), and alcohol and carboxylate salt are formed.
For example:
Ethyl propanoate + sodium hydroxide → sodium propanoate + ethanol
CH₃CH₂COOCH₂CH₃ + NaOH → CH₃CH₂COONa+ + CH₃CH₂OH
The carboxylate ion is resistant to attack by nucleophiles, and so the reaction is not reversible.
Vegetable oils and Animal Fats
Fatty acids contain long hydrocarbon chains of carboxylic acids.
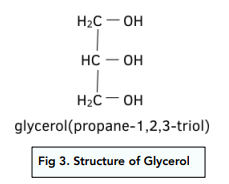
Fatty acids combine with glycerol (propane-1,2,3 triol) to make esters which are fats and oils.
The structure of glycerol is:
An ester link is made between glycerol and the fatty acid as shown below.
An example is shown in the diagram.
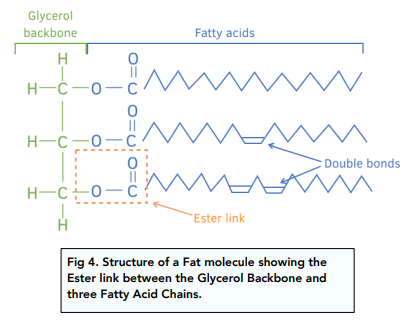
The properties of a fat or oil depends on the nature of the fatty acid chains it contains. Fatty acid chains can be:
- Unsaturated – If there are double bonds within the chain, the fatty acid chain is said to be unsaturated. Unsaturated fatty acids are more common in vegetable oils. They have lower boiling points and are liquid at room temperature.
- Saturated – If there are no double bonds within the chain, the fatty acid chain is said to be saturated. Saturated fatty acids are more common in animal fats. They have higher boiling points and are more solid at room temperature.
Hydrolysis of Fats and Oils
Like all esters, animal fats and vegetable oils can be broken apart by hydrolysis in alkaline conditions.
When fats or oils are heated with sodium or potassium hydroxide, the product made is soap.
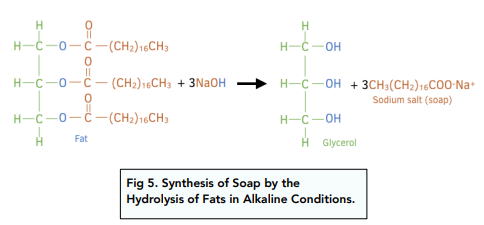
The soap contains a hydrophobic (fatty acid chain) end and a hydrophilic end (carboxylate ion).
The hydrophobic end mixes with grease and the hydrophilic end mixes with water.
With both types of group in the same molecule, grease and water can be mixed and washed away together.
Biodiesel
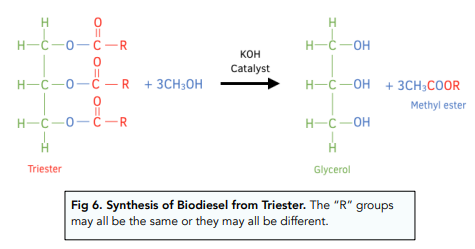
Biodiesel is made from vegetable oil (particularly rape seed oil).
The vegetable oil is reacted with methanol, using a strong alkali (e.g. potassium hydroxide) as a catalyst.
This breaks the vegetable oil apart to produce a mixture of methyl esters.
This mixture of methyl esters is called biodiesel.
For example:
Worked example: Vegetable oil is made by reacting one mole of vegetable oil with excess methanol. One mole of an ester with a formula C₁₈H₃₂O₂, one mole of an ester with a formula C₂0H₃₆O₂ and one mole of ester with a formula C₁₉H₃₄O₂ are formed from the reaction.
Draw the structure of the original vegetable oil showing the ester links.
Answer:
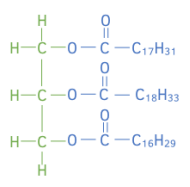
Explanation:
The esters formed are methyl esters.
Take the formula of each ester, and take away a H₃COOC- group to get the formula of the “R” group for each of the three chains.
C₁₈H₃₂O₂ take away H₃COOC = C₁₆H₂₉
C₂0H₃₆O₂ take away H₃COOC = C₁₈H₃₃
C₁₉H₃₄O₂ take away H₃COOC = C₁₇H₃₁
Add each of the “R” groups to the glycerol molecule to obtain the structure of the vegetable oil.





Still got a question? Leave a comment
Leave a comment