Transition Metals - Ligand Substitution Reactions (A-Level Chemistry)
Ligand Substitution Reactions
Ligand Substitution
Substitution by Water Ligands
If you dissolve any transition metal salt in water, the central transition metal atom or ion becomes surrounded by water ligands.
Complexes with water are called metal-aqua complex ions. They are octahedral and have a co-ordination number of 6.
For example:
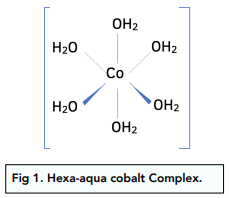
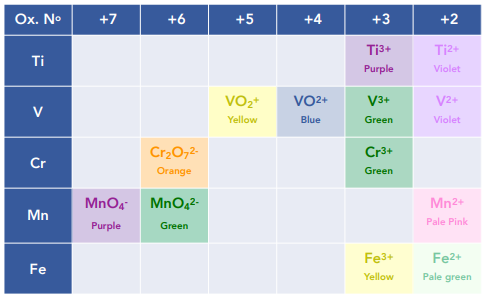
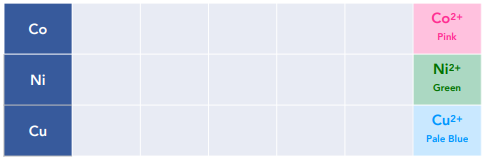
Solutions containing aqueous complexes have particular colours which can help to identify which transition metal is present.
Substitution by Other Ligands
These water ligands can be replaced by other ligands. This occurs because:
- Other ligands form stronger co-ordinate bonds than water
- Other ligands are present in higher concentrations
Complete and Incomplete Substitution
Complete ligand substitution occurs when all the original ligands are replaced.
Incomplete ligand substitution occurs when only some of the original ligands are replaced.
Incomplete ligand substitution occurs if the partially substituted complex is energetically more stable than either the original complex or completely substituted complex.
Ligand Substitution: No Change in Co-ordination Number
If the ligands are of a similar size (e.g. water and ammonia), the cordination number and shape of the complex formed remain the same.
Ligand substitution is a reversible process.
Example: Ligand Substitution in Cobalt (II)
Using cobalt (II) as an example, the overall equation shows the complete ligand substitution reaction with ammonia.
[Co(H₂O)₆]²⁺(aq) + 6NH₃ (aq) ⇌ [Co(NH₃)₆]²⁺(aq) + 6H₂O(l)
We see a colour change in this reaction from pink [Co(H₂O)₆]²⁺ (aq) to orange/ yellow [Co(NH₃)₆]²⁺ (aq)
This reaction happens in two steps. First, ammonia acts as a base and removes a proton from two of the water molecules. The water molecules have been deprotonated in a typical acid-base reaction to form OHions.
[Co(H₂O)₆]²⁺ (aq) + 2NH₃(aq) ⇌ [Co(OH)₂(H₂O)₄](s) + 2NH₄⁺(aq)
This results in the formation of a neutral complex, which is insoluble as it has no charge. This is seen as a blue precipitate of hydrated cobalt(II) hydroxide. If left standing, the precipitate will become oxidised and turn brown.
The addition of more concentrated ammonia results in complete ligand exchange as shown above. The blue precipitate dissolves to form a pale yellow solution.
The higher concentration of ammonia shifts the equilibrium position to favour the formation of [Co(NH₃)₆]²⁺
[Co(OH)₂(H₂O)₄](s) + 6NH₃(aq)⇌ [Co(NH₃)₆]²⁺ (aq) + 4H₂O(l) + 2OH⁻ (aq)
Both [Co(H₂O)₆]²⁺ and [Co(NH₃)₆]²⁺ are octahedral complexes with a coordination number of six.
Example: Ligand Substitution in Copper (II)
Using copper (II) as the central transition metal ion, only partial substitution occurs, as only four of the water ligands are replaced. The overall equation is:
[Co(H₂O)₆]²⁺(aq) + 6NH₃(aq) ⇌ [Cu(NH₃)₄(H₂O)₂]²⁺(aq) + 4H₂O(l)
The [Cu(H₂O)₆]²⁺ is pale blue, whilst ₂ is a deep blue solution
Looking at the reaction in more detail, it follows the same steps as for cobalt (II):
- Deprotonation. Ammonia first acts as a base and remove two protons from two water ligands. This forms a neutral complex so that a pale blue precipitate of [Cu(OH)₂(H₂O)₄](s) is observed.
- Ligand Substitution. Adding more concentrated ammonia causes the blue precipitate to dissolve to form the deep blue [Cu(NH₃)₄(H₂O)₂]²⁺
The deprotonation reaction can be reversed by adding an acid. The acid will protonate the hydroxide ligands so that the soluble metal-aqua ions reform and the precipitate redissolves.
Ligand Substitution: Change in Co-ordination Number
When water molecules are replaced by larger ligands, for example chloride ions, there is a change in co-ordination number and shape. This is because only four chloride ions can fit around the central transition metal ion.
The complex formed is tetrahedral in shape.
Concentrated hydrochloric acid provides a high concentration of chloride ions. This is used to carry out ligand exchange reactions for chloride ions.
Here are some examples of ligand substitution with a change in coordination number and shape.

Chelate Effect
When bidentate and multidentate substitute monodentate ligands, a more stable complex is formed. This is called the chelate effect.
When ligand exchange reactions occurs, the strength of the ligand bonds broken are very similar to the strength of the new bonds being made. This means the enthalpy changes, ∆H, for many ligand substitution reactions are very small.
The explanation for the increased stability is due mostly to an increase in entropy, ∆S. There is more disorder in the products (increased entropy) as there are more product molecules formed.
Example: EDTA ⁴⁻ replacing water ligands
Consider the reaction where an EDTA ⁴⁻ ligand replaces water ligands.
[Cu(H₂O)₆]²⁺ (aq) + EDTA⁴⁻(aq) ⇌ [Cu(EDTA)]²⁻(aq) + 6H₂O(l)
There are two reactant particles and seven product particles. This increase in product particles greatly increases the entropy, ∆S.
The Gibb’s free energy ∆G, will be negative as the change in entropy, ∆S, is positive and the change in enthalpy, ∆H, is small.
∆G = ∆H – T∆S
This means the equilibrium for the reaction will be far to the right.
Therefore, multidentate and bidentate ligands form more stable complexes than monodentate ligands.
Haemoglobin
Our red blood cells contain the protein known as haemoglobin. This is responsible for carrying oxygen from our lungs to all the cells in our body.
Haemoglobin contains the transition metal ion, Fe²⁺, at its centre. This is responsible for making the blood cells to be coloured red.
Part of the structure of haemoglobin is a transition metal complex called haem.
Structure of Haem
The structure of haem is shown in the diagram below:
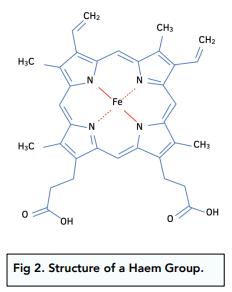
The co-ordination number of this complex is six and the shape is octahedral.
Four of the ligands are formed with nitrogen atoms as shown in colour.
There is a fifth nitrogen atom below the plane of this structure which is part of a protein called globin. This forms the fifth ligand.
The sixth ligand is made with oxygen molecules present in high concentrations, as in the lungs. This forms the complex oxyhaemoglobin.
The co-ordinate bond between Fe²⁺ and oxygen molecules is weak.
Haem in Low Oxygen Concentrations
In cells with low concentrations of oxygen, the co-ordinate bond with oxygen breaks apart, and the oxygen ligand is replaced with a water ligand.
The oxygen is given up to the cells where it is needed.
Haem and Carbon Monoxide
Some molecules form stronger co-ordinate bonds with the Fe²⁺ in haem compared to oxygen. An example is carbon monoxide.
Carbon monoxide is formed from incomplete combustion of hydrocarbons.
The carbon monoxide ligand forms a strong co-ordinate bond in the place of the oxygen ligand. It forms a complex called carboxyhaemoglobin. The carbon monoxide is strongly bonded to the Fe²⁺ ion and it not easily removed.
Therefore the haemoglobin molecule can no longer carry oxygen to the body cells. Even in low concentrations of carbon monoxide, the effects can be fatal if not treated.
Stability Constant
Ligands in a complex can be partially or fully exchanged for other ligands provided that the newly formed complex is more stable than the original.
The stability constant (Kstab) for a transition metal ion complex is the equilibrium constant for the formation of the said ion in a solvent from its constituent ions or molecules.
For example:
[Cu(H₂O)₆]²⁺(aq) + 6NH₃(aq) ⇌ [Cu(NH₃)₄(H₂O)₂]²⁺(aq) + 4H₂O(l)
Therefore:
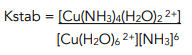
Kstab is used as a measure of stability: the higher the Kstab of a complex, the more stable it is.
- High Kstab – Position of equilibirum lies further to the right hand side. The product is more stable.
- Low Kstab – Position of equilibrium lies further to the left hand side. The reagent is more sable.
Kstab can therefore be used to predict whether a ligand substitution reaction will happen by comparing the Kstab values of both complexes.
FAQs
Transition metals are a group of elements found in the middle of the periodic table, typically characterized by having high melting and boiling points, as well as multiple oxidation states.
Ligand substitution reactions are chemical reactions where one or more ligands (molecules or ions that bind to the central metal ion) are replaced by new ligands.
Ligand substitution reactions in transition metals occur as a result of the coordination complex (a central metal ion surrounded by ligands) being unstable. The reaction occurs to restore the stability of the coordination complex by replacing the old ligands with new ones.
The role of the ligands in ligand substitution reactions is to coordinate (bind) to the central metal ion in the coordination complex. The stability of the coordination complex depends on the number and type of ligands bound to the central metal ion.
The rate of ligand substitution reactions can be affected by a variety of factors, including the nature of the ligands, the nature of the metal ion, the temperature, and the presence of any catalysts.
Inner-sphere ligand substitution reactions occur when the metal-ligand bonds are broken and new bonds are formed. Outer-sphere ligand substitution reactions, on the other hand, occur without breaking the metal-ligand bonds, but instead, the new ligands interact with the coordination complex without forming new bonds.
One example of a ligand substitution reaction is the reaction between iron(II) chloride and sodium cyanide to form the coordination complex, iron(II) cyanide. In this reaction, the chloride ions are replaced by cyanide ions.
Simple ligand substitution reactions involve the replacement of one or two ligands in the coordination complex, whereas complex ligand substitution reactions involve the replacement of three or more ligands.
Ligand substitution reactions are important in a variety of real-world applications, including the production of pigments and dyes, the extraction of metals from ores, and in the synthesis of drugs and catalysts.
Understanding ligand substitution reactions is important for A-Level Chemistry students as it provides a deeper understanding of the coordination chemistry of transition metals. This knowledge is crucial for further studies in chemistry and related fields, as well as for understanding the role of transition metals in various real-world applications.






Still got a question? Leave a comment
Leave a comment