Chemical Equilibria and Le Chatelier's Principle (A-Level Chemistry)
Chemical Equilibria and Le Chatelier’s Principle
Reversible Reactions
A reversible reaction is a reaction that can occur in both directions. The reactants can form the products but then the products can also form the reactants. The direction in which the reaction goes depends on the conditions. Understanding these principles is crucial for optimizing processes in various industries, a skill often developed and applied during Chemical Engineering work experience.
For example, The Haber Process is an example of a reversible reaction which has the following equation: N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
The double headed arrow ⇌ shows that this reaction is reversible meaning it can go either both directions.
The change from left to right in the equation is known as the forward reaction. The change from right to left is the backwards reaction.
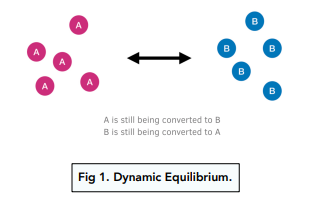
Dynamic Equilibrium
A dynamic equilibrium is formed when the forward reaction is happening at the same rate as the backward reaction. At equilibrium, the quantities of everything present (concentrations of reactants and products) in the mixture remain constant, although the reactions are still continuing.
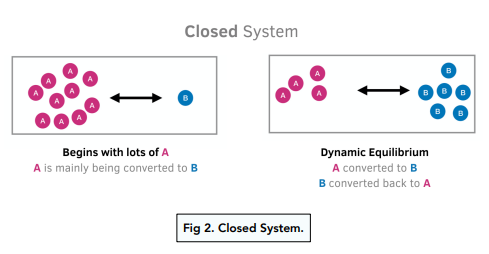
Closed Systems
A dynamic equilibrium only occurs when you have a reversible reaction in a closed system. A closed system is a system where nothing can be added to the system or taken away from it, apart from energy.
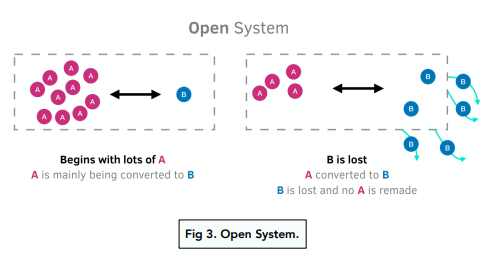
Applying Le Chatelier’s Principle
Le Chatelier’s Principle
Le Chatelier’s principle states that:

This means that if either the temperature, pressure or concentration of a reversible reaction is changed, the position of equilibrium will be altered to counteract the change.
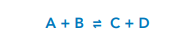
Rules for Le Chatelier’s principle:
• More reactants will be produced if the position of the equilibrium moves to the left.
• More products will be produced if the position of the equilibrium moves to the right.
You need to be able to use Le Chatelier’s principle predict qualitatively the effects of changes in temperature, pressure and concentration on the position of equilibrium in homogeneous reactions:
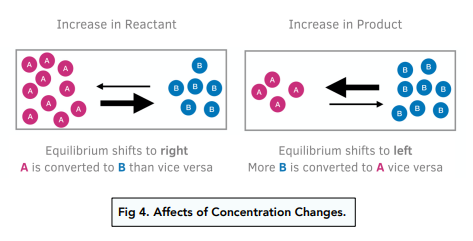
Change of Concentration (For Reactions in Solution)


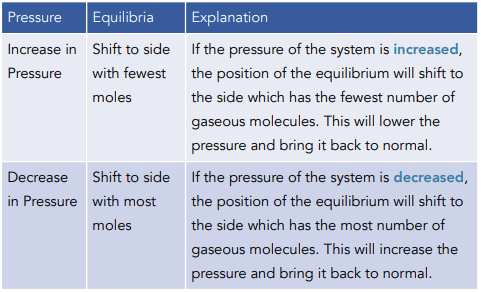
Change of Pressure (For Gaseous Reactions)
Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be.
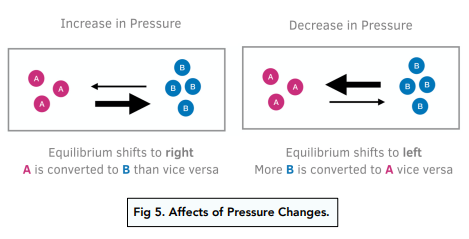
e.g. Let’s take the reaction: N2O4 (g) ⇌ 2NO2 (g)
There are two gas molecules on the right hand side and one gas molecule on the left hand side. If the pressure of the system is increased, the position of the equilibrium will shift to the side with the fewest number of moles which is the left hand side.
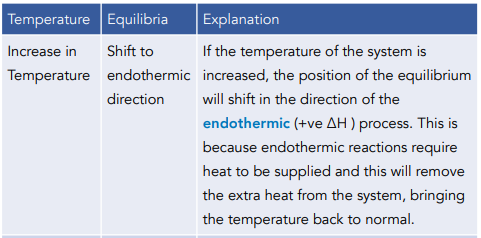
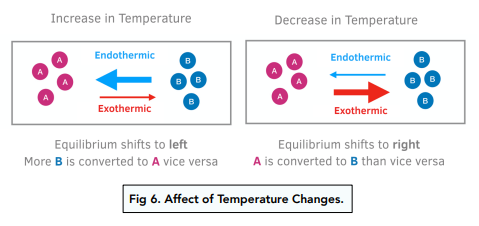
Change of Temperature

e.g. Let’s take the reaction: CaO (s) + H2O (l) ⇌ Ca(OH)2 (aq) ∆H = – 82 kJmol-1
The forward reaction has a -ve ∆H therefore it is an exothermic reaction. This means the backward reaction is endothermic. If the temperature is decreased, the position of equilibrium will shift in the direction of the forward reaction. If the temperature is increased, the position of equilibrium will shift in the direction of the backward reaction.
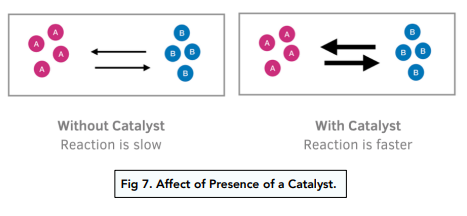
Catalysts
Adding a catalyst to a reaction has has no effect on the position of equilibrium because a catalyst speeds up the forward and backward reaction by the same amount. However, adding a catalyst increases the rate at which a reaction reaches dynamic equilibrium. This is because it increases the speed of the forward and backward reaction. The yield is also not affected.
Chemical equilibria refers to the state in a chemical reaction where the rates of the forward and reverse reactions are equal and the concentration of the reactants and products remains constant.
Le Chatelier’s principle is a fundamental principle in chemistry that states that a chemical system at equilibrium will adjust to counteract any change imposed upon it. This principle can be used to predict the effect of changes in temperature, pressure, or concentration on a chemical system in equilibrium.
Le Chatelier’s principle can be used to understand how changes in temperature, pressure, and concentration affect the position of a chemical equilibrium. If a stress is applied to a chemical system at equilibrium, the system will respond in such a way as to counteract the stress and reestablish the original equilibrium.
Yes, changes in temperature can affect chemical equilibria. If the temperature of a chemical system in equilibrium is increased, the reaction will shift in the direction that absorbs heat, while if the temperature is decreased, the reaction will shift in the direction that releases heat.
Yes, changes in pressure can affect chemical equilibria. If the pressure of a gas-phase chemical system in equilibrium is increased, the reaction will shift in the direction that consumes gas, while if the pressure is decreased, the reaction will shift in the direction that produces gas.
Yes, changes in concentration can affect chemical equilibria. If the concentration of one of the reactants or products in a chemical system in equilibrium is increased, the reaction will shift in the direction that consumes that species, while if the concentration is decreased, the reaction will shift in the direction that produces that species.
Le Chatelier’s principle is important in the chemical industry because it can be used to optimize reaction conditions for maximum yield and efficiency. For example, the principle can be used to determine the ideal temperature, pressure, and concentration conditions for a chemical reaction to maximize the production of a desired product.
Le Chatelier’s principle can be applied to real-world problems such as air pollution control and waste treatment. The principle can be used to predict the effects of changes in temperature, pressure, and concentration on the reaction rates in these systems, allowing for the optimization of reaction conditions to achieve desired outcomes.





Still got a question? Leave a comment
Leave a comment