Acids and Bases - pH of Strong Bases (A-Level Chemistry)
pH of Strong Bases
Calculating the pH of Strong Bases
Using Kw to Work Out the pH of Strong Bases
Strong bases fully dissociate in solution to release (OH- ) ions. You can calculate the pH of a strong base using the Kw equation, as it sets up a relationship between [OH- ] and [H+].
Worked Example: Calculate the pH of a solution of 0.2 mol dm-³ of Ba(OH)2 at 25 °C
Answer:
Step 1: Calculate the concentration of hydroxide ions.
Two moles of hydroxide ions are released from each mole of barium hydroxide. The concentration of OH- ions = 0.2 x 2 = 0.4 mol dm-³
Step 2: Use the value of Kw to calculate the concentration of H+ ions
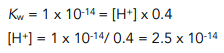
Step 3: Use the concentration of H+ ions to work out the pH.
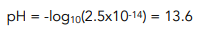
Worked Example: Calculate the pH of the solution formed when 12.00 cm³ of 0.2 mol dm-³ hydrochloric acid is added to 24.00 cm³ of 0.2 mol dm-³ barium hydroxide solution at 298 K
Answer:
Step 1: Work out the moles of acid and base
Moles of acid = volume x concentration = 12/1000 0.2 = 2.4 x 10-³ mol
Moles of base = volume x concentration = 24/1000 x 0.2 = 4.8 x 10-³ mol
Step 2: Use the balanced equation to identify which substance is in excess
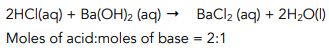
There are 2.4 x 10-³ moles of acid, so the amount of base reacting = 1.2 x 10-³ mol.
Therefore moles of base remaining in the solution = (4.8-1.2) x 10-³ = 3.6 x10-3 mol
Step 3: Work out the concentration of OH- ions remaining
Moles of barium hydroxide = 3.6 x 10-³
Therefore moles of OH- ions = 2 x 3.6 x 10-³ = 7.2 x 10-³ mol
Total volume in the solution = 24+ 12 = 36.00 cm³ = 0.036 dm³
Concentration of OH- ions = moles/ volume = 7.2 x 10-³/0.036 = 0.2 mol dm-³
Step 4: Use Kw to work out the H+ concentration
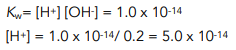
Step 5: Use [H+] to work out pH






Still got a question? Leave a comment
Leave a comment