Aldehydes and Ketones - Reactivity of Carbonyl Compunds (A-Level Chemistry)
Carbonyl Compound Reactivity
Nucleophilic addition reactions of aldehydes and ketones
Reaction Mechanism
The permanent dipole on the carbonyl functional group results in the carbon atom having a partial positive charge, which leaves it open to attack by nucleophiles.
Carbonyl compounds can therefore undergo nucleophilic addition reactions, where a nucleophile gets added across the C=O double bond.
The reaction happens in two steps:
1. Nucleophilic attack. The lone pair of electrons from the nucleophile attacks the “????+” C atom on the carbonyl group.
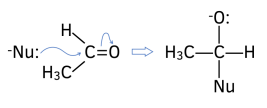
2. New O-H bond forms. The lone pair of electrons from the negative oxygen atom attracts and forms a bond with H+ ion from the solvent (either dilute acid or water).
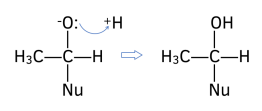
Example: Reduction of aldehydes and ketones
We previously learnt that primary alcohols can be oxidised to form aldehydes and that secondary alcohols are oxidised to make ketones.
Many reducing agents will reduce aldehydes and ketones back to their respective alcohols.
Common examples of reducing agents include sodium tetrahydridoborate (III) (NaBH₄) – also referred to as or sodium borohydride – and lithium tertrahydroaluminate (LiAlH₄) – also referred to as lithium aluminum hydride – in dry ether.
In the structure of NaBH4, the boron atom forms a dative covalent bond with one of the hydrogen atoms. In an aqueous solution, the hydride ion H:- is formed.
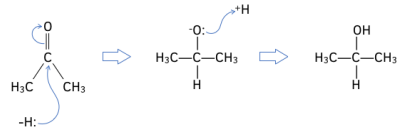
The reduction of carbonyl compounds is a typical nucleophilic addition reaction. The steps to the reaction are as follows:
1. This H:- ion acts as a nucleophile. The lone pair of electrons from the H:- ion attacks the “????+” C atom on the carbonyl group.
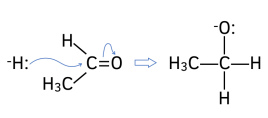
2. New O-H bond forms. The lone pair of electrons from the negative oxygen atom attracts and forms a bond with H+ ion from the solvent (either dilute acid or water).
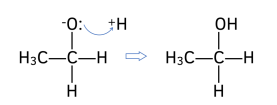
Worked Example: Draw the reaction mechanism for the reaction of propanone with NaBH₄. Give the IUPAC name for the product formed.
Answer: Propanol is made.
Example: Reaction of carbonyl compounds with CN⁻
Carbonyl compounds can undergo nucleophilic addition by cyanide ions. The products formed are called hydroxynitriles.
At room temperature and pressure, aldehydes and ketones react with potassium cyanide followed by dilute hydrochloric acid.
The dilute acid supplies H⁺ ions which are needed in the reaction mechanism.
This reaction mechanism is important products in organic synthesis, as the carbon chain has increased in length by one carbon atom.
The reaction mechanism for the reaction between propanone and KCN, followed by dilute acid is:
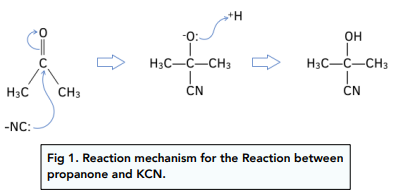
The product is called 2-hydroxy-2-methylpropanenitrile.
Worked Example: Name the product formed when ethanal reacts with potassium cyanide, followed by dilute acid.
Answer: 2-hydroxypropanenitrile
Formation of racemic mixtures
When aldehydes or unsymmetrical ketones react with KCN followed by dilute acid, they form mixtures of enantiomers. When there are equal amounts of enantiomers, a racemic mixture is formed.
The reason this is possible is because the C=O group is planar, therefore the :CN- ion can attack from either above or below the molecule.
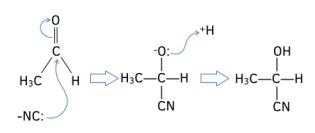
Worked Example: Identify the aldehyde or ketone which has formed the following enantiomer. Draw a reaction mechanism for the reaction.
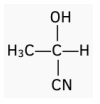
Answer: Ethanal





Still got a question? Leave a comment
Leave a comment