Atomic Structure - Electrons in Atoms (A-Level Chemistry)
Electrons in Atoms
Electron Shells and Sub-Shells
Definitions
Electron shells are regions of space at different distances from the nucleus which can contain up to a certain number of electrons of a fixed energy level.
A sub-shell is one or more orbitals in the same shell which have the same energy levels.
An orbital is the volume of space where an electron is most likely to be found.
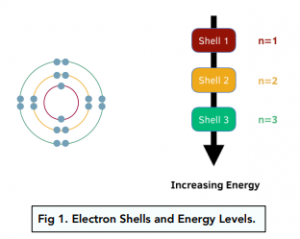
Electron Shells and Energy Levels
- Electrons move in electron shells around the central nucleus. When electrons move around the nucleus, they don’t all move in one orbit. Instead, they are found in different shells, which represent specific energy levels. The shells are found at different distances from the nucleus, so electrons in different shells have different energy levels.
- Electrons have fixed energies. Each electron shell has a particular energy, and electrons can only have one of the fixed energy levels of the shells – they cannot have intermediate energy values that don’t lie on one of the energy levels. The fixed energy of an electron is the amount of energy needed to move electrons from one energy level to another.
- An electron shell that is further from the nucleus has a higher energy level. The energy level of electron shells increases as distance from the nucleus increases, as this means there is a decreased interaction with the nucleus.
- Principal quantum number is the number given to each electron shell. Each electron shell has a principal quantum number. The shell closest to the nucleus has quantum number 1, the shell second closest has quantum number 2, and so on. So the further away a shell is from the nucleus, the higher the principle quantum number.
Sub-Shells
- Electron shells are divided into sub-shells which each have a different energy. We learnt above that electrons are split into shells, each with their own energy level. Well, electrons in each shell can be further split into mini sub-shells, meaning that the electrons in the same shell do not all have the same energy level.
- There are 4 sub-shells. There are 4 different sub-shells – s, p, d and f. Different sub-shells have different shapes.
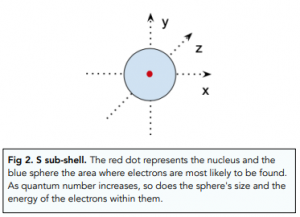
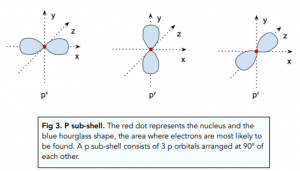
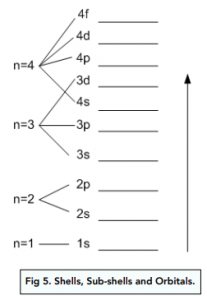
- Lower energy sub-shells are closer to the nucleus. Within each shell, we can have multiple sub-shells. The lower the energy of a sub-shells, the closer it will be to the nucleus. For example:
- The s sub-shell has the lowest energy because it contains electrons that are closest to the nucleus.
- The f sub-shell has the highest energy because it contains electrons that are furthest from the nucleus.
- As principal quantum number increases, sub-shells begin to overlap. As we move to shells with higher quantum numbers (i.e. further away from the nucleus), the sub-shells begin to overlap. This is because the energy gap between successive sub shells get smaller and smaller. For example, sub shell 3d has a higher energy level than sub shell 4s, even though shell 3 is an overall lower energy level than shell 4.
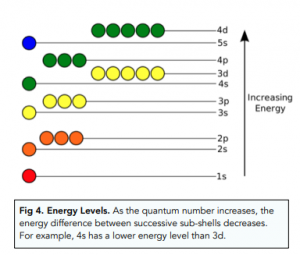
Electrons in Sub-Shells
- Each sub-shells can hold a specific number of electrons. In each subshell, the electrons are held in orbitals. Each orbital can hold 2 electrons, and each sub-shell can have a variable number of orbitals. For example, s sub-shells have 1 orbital and so can hold 2 electrons, whilst f sub-shells have 7 orbitals and so can hold 14 electrons.
You need to know the maximum number of electrons and number of orbitals found in each sub-shell, as shown below:
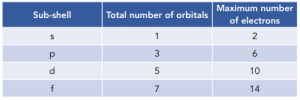
Electrons in Shells
- Each shell can hold a specific number of electrons. Using the information in the table above, we can then calculate the number of electrons that each shell can hold. This is shown in the table below:
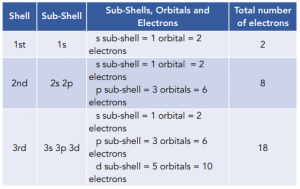
Summary
- The 1st shell has 1 sub-shell
- The 2nd shell has 2 sub-shells
- The 3rd shell has 3 sub-shells
- The 4th shell has 4 sub-shells
- The S sub-shell contains 1 orbital.
- The P sub-shell contains 3 orbitals
- The D sub-shell contains 5 orbitals
- The F sub-shell contains 7 orbitals.
Orbitals
Each orbital contains a maximum of 2 electrons which spin in opposite directions. Each orbital contains two electrons which spin in opposite directions. As the electrons spin on their axes, the moving charge results in formation of a magnetic field. In each orbital, one electron spins up and the other spins down.
Orbitals within each sub-shell have the same energy level. Whilst different shells have different energy levels, and different sub-shells have different energy levels, different orbitals within the same sub-shell have the same energy level.
The atomic structure consists of a nucleus made up of protons and neutrons, surrounded by electrons that orbit the nucleus.
Electrons are negatively charged subatomic particles that orbit the nucleus of an atom. They are responsible for chemical bonding and the electrical conductivity of materials.
Electrons are arranged in shells or energy levels around the nucleus. Each shell can hold a maximum number of electrons, and as you move further from the nucleus, the energy of the shells increases.
The electronic configuration of an atom describes the arrangement of electrons in its various energy levels or shells. It is represented by a series of numbers and letters that indicate the number of electrons in each shell.
An orbital is a region of space around the nucleus where an electron is most likely to be found. Each orbital can hold a maximum of two electrons with opposite spins.
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each electron in an atom must occupy a unique orbital.
Valence electrons are the electrons in the outermost shell of an atom. They are responsible for chemical bonding and determine the reactivity of the element.
Electrons can move between energy levels by absorbing or emitting energy in the form of photons. When an electron absorbs energy, it moves to a higher energy level, and when it emits energy, it moves to a lower energy level.
The Aufbau Principle states that electrons fill the lowest energy level first before moving to higher energy levels. This means that the electronic configuration of an atom can be determined by following a specific order of filling the shells with electrons.
Electron configuration notation is a shorthand way of representing the electronic configuration of an atom. It consists of the energy level, followed by the letter representing the orbital and the number of electrons in that orbital. For example, the electron configuration of carbon is 1s²2s²2p².





Still got a question? Leave a comment
Leave a comment