Amount of Substance - Empirical and Molecular Formulae (A-Level Chemistry)
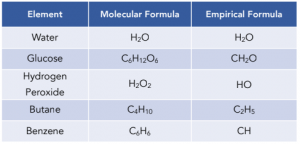
Empirical and Molecular Formulae
Formulas As Ratios
Definitions
The empirical formula is the simplest whole number ratio of atoms of each element in a compound.
The molecular formula is the actual number of atoms of each element in a compound.
The empirical mass is the total mass of all the atoms in the empirical formula.
Empirical vs. Molecular Formula
A certain number of empirical units make up the molecular formula. For example, the molecular formula of hydrogen peroxide is H2O2, but the empirical formula is HO because the simplest ratio of hydrogen to oxygen atoms is 1:1.
Calculating Molecular Formula from Empirical Formula and Mr
You may be asked to calculate molecular formula from the empirical formula and relative molecular mass.
Practice Question: The empirical formula of a molecule is CH2O. It has a relative molecular mass (Mr) of 180. What is its molecular formula?
1. Firstly work out the empirical mass of the molecule
Empirical mass = 12 + (1 x 2) + 16 = 30
2. Divide the molecular mass by the empirical mass.
Next look at the Mr you are given in the question and compare it with the empirical mass you calculated in step 1. Compare the values by dividing the Mr by the empirical mass, and the value you achieve will tell you how many empirical units make up the molecule.
Mr = 180
Empirical mass = 30
Mr ÷ Empirical mass = 180 ÷ 30 = 6
This means there are 6 empirical units in the molecule CH2O.
3. Multiply the empirical formula by the number of empirical units.
Finally to calculate the molecular formula by multiplying the empirical formula by the number of empirical units found in the molecule.
In this case we multiply the empirical formula which is CH2O by 6 as there are 6 empirical units.
When we multiply the empirical formula, we multiply the number of atoms of each element in the molecule by 6.
Therefore the molecular formula is C6H12O6. The molecule is therefore glucose.
Calculating Empirical Formula from % Composition by Mass
You can calculate the empirical formula of a molecule from the percentage composition of the elements found in the molecule.
Practice Question: The percentage composition of a particular compound, by mass, is 64.8% Carbon, 13.62% Hydrogen, and 21.58% Oxygen. What is its empirical formula? (Ar values are as follows, C = 12, H = 1, O = 16)
1. First change all the percentages to grams, and assume 100g of the compound is present.
Carbon = 64.8% = 64.8g
Hydrogen 13.62% = 13.62g
Oxygen = 21.58% = 21.58g
2. Next calculate the number of moles of each element in the compound.
We need to use the equation that was introduced earlier.

Moles of C = 64.8g / 12 = 5.4
Moles of H = 13.62g / 1 = 13.62
Moles of O = 21.58g / 16 = 1.349
3. Then find the empirical formula by dividing the number of moles of each element by the lowest number of moles
In this case the lowest number of moles is that of oxygen which is 1.349 moles
Carbon = 5.4/1.349 = 4
Hydrogen = 13.62/1.349 = 10
Oxygen = 1.349/1.349 = 1
Therefore the empirical formula is: C4H10O
Calculating Empirical Formula from % by Mass
You can also calculate the empirical formula of a molecule from masses found from experimental data.
The question below is about a hydrocarbon. Remember, a hydrocarbon is a compound that consists of only the elements carbon and hydrogen.
Practice Question: What is the empirical formula of a hydrocarbon if 4.4g of CO2 and 3.6g of H2O are formed when it is burnt in excess oxygen? (Ar values are as follows, C = 12, H = 1, O = 16)
1. Firstly calculate the number of moles of each of the products CO2 and H2O

Mr of CO2 = 12 + (16 x 2) = 44
Mr of H2O = (1 x 2) + 16 = 18
Number of moles of CO2 = 4.4/44 = 0.1 moles
Number of moles of H2O = 3.6/18 = 0.2 moles
2. Next work out how many moles of carbon and hydrogen atoms are found in the hydrocarbon
In 1 mole of CO2, 1 mole of carbon atoms are present
In 1 mole of H2O, 2 moles of hydrogen atoms are present
This means that the hydrocarbon contains:
0.1 x 1 = 0.1 carbon atoms
0.2 x 2 = 0.4 hydrogen atoms
3. Then calculate the empirical formula of the hydrocarbon
We can calculate the empirical formula of the hydrocarbon by forming the simplest whole number ratio of carbon to hydrogen atoms in the hydrocarbon.
Ratio of Carbon : Hydrogen = 0.1 : 0.4
Therefore the empirical formula is CH4. The hydrocarbon is methane.
Calculating Empirical Formula from Volume Data
You can also calculate the empirical formula of a molecule from volumes found from experimental data.
Practice Question: 150cm3 of CO2 are formed when 50cm3 of a hydrocarbon in gaseous form is burnt with exactly 250cm3 of oxygen. What is the empirical formula of the hydrocarbon (CxHy)?
1. Firstly calculate the mole ratio.
CxHy + O2 —> CO2 + H2O
According to Avogadro’s Law which we previously looked at, same volumes of gasses at the same temperature and pressure, will contain the same number of moles
Therefore the ratio of volumes will be the same as the ratio of moles. This ratio can be used as the reaction coefficients.
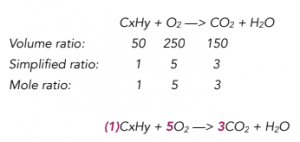
2. Next work out how many moles of carbon and hydrogen atoms are found in the hydrocarbon.
If you look at the unbalanced equation, all carbon atoms in the hydrocarbon are used to make CO2. We have 3 carbon atoms that make part of CO2 so x=3
C3Hy + 5O2 —> 3CO2 + H2O
3 mol out of the 5 mol of O2 are used to make CO2. There are 3 mol of O2 (4 mol of O atoms) left to make water so the the coefficient of water is 4.
C3Hy + 5O2 —> 3CO2 + 4H2O
All of the hydrogen atoms in the hydrocarbon are used to make water. We have 8 hydrogen atoms in total that make part of water so y=8.
C3H8 + 5O2 —> 3CO2 + 4H2O
3. Then calculate the empirical formula of the hydrocarbon
In this example the ratio to carbon to hydrogen that make up the hydrocarbon already is in its simplest form. Therefore, the empirical formula of the hydrocarbon is C3H8.
FAQs
The amount of substance in chemistry refers to the amount of a substance that is present in a sample. It is usually measured in units of moles.
Empirical and molecular formulas are two types of chemical formulas used to describe the composition of a compound.
The empirical formula is the simplest whole-number ratio of atoms in a compound. For example, the empirical formula of glucose is CH2O, which indicates that the ratio of carbon to hydrogen to oxygen atoms in glucose is 1:2:1. The empirical formula can be determined experimentally from the mass percent composition of the compound.
The molecular formula, on the other hand, gives the actual number of atoms of each element in a molecule of the compound. For example, the molecular formula of glucose is C6H12O6, which indicates that a molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
The molecular formula can be determined from the empirical formula and the molar mass of the compound. If the molar mass of the compound is known, the molecular formula can be calculated by multiplying the empirical formula by a whole number that gives the correct molar mass.
Empirical and molecular formulas provide important information about the composition of a compound and are used in a variety of applications, including chemical synthesis, industrial production, and pharmaceutical research.
Empirical formula is the simplest whole-number ratio of atoms present in a molecule, whereas the molecular formula is the actual number of atoms present in a molecule.
To calculate the empirical formula of a compound, you need to determine the simplest whole-number ratio of atoms in the compound. This can be done using experimental data on the mass percent composition of the compound.
The general steps for calculating the empirical formula are:
Convert the mass percent of each element to mass (grams or milligrams) of the element in a known mass of the compound. For example, if a 1.00-gram sample of a compound contains 0.40 grams of carbon, the mass percent of carbon in the compound is 40%.
Convert the mass of each element to moles using the atomic weight (or molecular weight) of the element. The atomic weight is the mass of one mole of atoms of the element, and is found on the periodic table. For example, the atomic weight of carbon is 12.01 g/mol.
Divide the number of moles of each element by the smallest number of moles calculated. This will give you the simplest whole-number ratio of atoms in the compound.
If necessary, multiply the subscripts in the empirical formula by a whole number to obtain whole numbers, but the subscripts should be as small as possible.
To find the molecular formula of a compound from its empirical formula, you need to know the molar mass of the compound. The molecular formula gives the actual number of atoms of each element in a molecule of the compound.
The general steps for finding the molecular formula from the empirical formula are:
Calculate the empirical formula mass by summing the atomic masses of all the atoms in the empirical formula.
Divide the molar mass of the compound by the empirical formula mass to obtain a whole-number multiplier (n).
Multiply the subscripts in the empirical formula by the multiplier (n) to obtain the molecular formula.
Knowing the empirical formula of a substance is important because it provides information about the simplest whole-number ratio of atoms present in a molecule, which can help in identifying the type of substance and its chemical properties.
The molecular formula is a multiple of the empirical formula. For example, if the empirical formula of a substance is CH2, its molecular formula could be CH4, C2H4, or C6H12.
The molecular formula can be determined by multiplying the empirical formula by a whole number that gives the actual number of atoms present in a molecule. The molecular formula can also be determined by other methods, such as mass spectrometry.
Knowing the empirical and molecular formulae of a substance has many practical applications, such as determining the chemical properties and reactivity of the substance, predicting the composition and properties of compounds, and developing new drugs and materials.






Still got a question? Leave a comment
Leave a comment