Transition Metals - Ligands (A-Level Chemistry)
Ligands in Complex Ions
Ligands in Complex Ions
A ligand is an atom or atom group that can donate a lone pair of electrons to a transition metal ion to form a complex through the formation of co-ordinate bonds.
All ligands have lone pairs of electrons.
Some common ligands are: water, ammonia, cyanide and chloride ions.

Monodentate Ligands
If ligands have one pair of electrons, they are called monodentate ligands. Examples include water, ammonia, chloride ions and cyanide ions.
Water and ammonia are uncharged, whereas chloride and cyanide have a -1 charge.
Bidentate Ligands
If ligands have two pairs of electrons, they are called bidentate ligands. Examples include 1,2-diaminoethane ![]() or the ethanedioate ion (C2O4²-)
or the ethanedioate ion (C2O4²-)
Their structures are shown below:
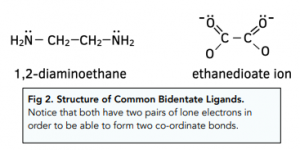
Multidentate Ligands
If ligands have many lone pairs of electrons, they are called multidentate ligands.
The example you need to be familiar with is the ethylenediaminetetracetate ion or EDTA 4- ion for short. This has six pairs of electrons.
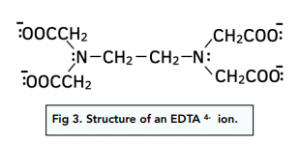
Co-ordination Number
The co-ordination number is the number of co-ordinate bonds formed with the central transition metal atom or ion.
Co-ordination Number: 6
Small ligands, like water and ammonia, have a co-ordination number of six, as six of these ligands can fit around the central transition metal atom or ion.
Bidentate ligands also have a co-ordination number of six. This is because three of these molecules can fit around the central transition metal ion.
EDTA 4- ion has six pairs of electrons. Each EDTA ion makes six co-ordinate bonds around the central transition metal atom. The coordination number is six.
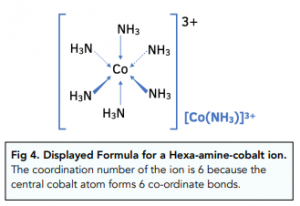
Co-ordination Number: 4
Larger ligands, like chloride ions, have a co-ordination number of four, as only four ligands can fit around the central transition metal atom or ion.
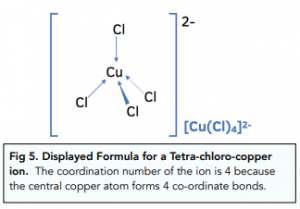
Transition metals are a group of elements located in the middle of the periodic table and are characterized by their ability to form multiple stable oxidation states. Some of the most well-known transition metals include iron, copper, and gold.
Ligands are chemical species that bond to transition metals to form coordination compounds. Ligands can be neutral molecules or ions, and they can bond to the metal through coordination bonds, which are covalent bonds between the metal and the ligand.
Ligands play a crucial role in transition metal chemistry by determining the oxidation state and coordination number of the metal, as well as the physical and chemical properties of the resulting coordination compound.
There are several types of ligands, including monodentate ligands, which bond to the metal through a single coordination bond, and polydentate ligands, which bond to the metal through multiple coordination bonds. Other types of ligands include chelating ligands, which form cyclic structures around the metal, and anionic ligands, which are negatively charged.
The color of transition metal compounds is often related to the energy required to promote an electron from a lower energy orbital to a higher energy orbital. Ligands can influence the energy levels of these orbitals, leading to changes in the color of the coordination compound.
Coordination number refers to the number of coordination bonds between a transition metal and its ligands, while oxidation state refers to the charge on the metal ion in a coordination compound. The coordination number and oxidation state of a transition metal can be influenced by the type and number of ligands present.
The study of transition metals and ligands is important in A-Level Chemistry because it provides a fundamental understanding of the properties and behavior of these elements, which are important in many industrial and biological processes. This knowledge is essential for understanding topics such as catalysis, the coordination of metal ions in biological systems, and the synthesis of coordination compounds.
Common techniques used to study transition metals and ligands include spectroscopy, such as ultraviolet-visible spectroscopy and infrared spectroscopy, and crystallography, which involves the study of the structure and properties of crystals. These techniques allow for the determination of the oxidation state and coordination number of transition metals and the identification of the ligands present in coordination compounds.






Still got a question? Leave a comment
Leave a comment