Amount of Substance - The Ideal Gas Equation (A-Level Chemistry)
The Ideal Gas Equation
Ideal Gas Equation
Ideal Gases
An ideal gas is a hypothetical gas whose volume varies in proportion to its temperature and in inverse proportion to its pressure. For a gas to be an ideal gas, as opposed to a real gas, its particles must follow certain rules:
- Lack of intermolecular forces. Gas molecules must neither repel or attract each other.
- Elastic collisions. The only interaction between gas molecules are elastic collisions. No energy is lost when particles collide against each other or against the surface of the container so that kinetic energy remains constant.
- Zero particle volume. Individual gas particles are so small in comparison to the empty space between them, that their volume is negligible.
No gas can behave exactly like an ideal gas but the lower their intermolecular forces, the more they can approach ideal behavior. Nobel gases for examples, have very low intermolecular forces and hence approach ideal behavior.
At room temperatures and pressures, many of the gases we care about can be assumed to behave ideally.
Understanding the Ideal Gas Equation
The ideal gas equation is used to calculate the number of moles found in a specific volume of a gas:

You need to know what each letter in the equation represents as well as the units used:
p = pressure (Pa)
V = volume (m3)
n = number of moles
R = 8.31 J K-1mol-1 (this is the gas constant that will be given to you in the exam)
T = temperature (K)
Unit Conversions for the Ideal Gas Equation
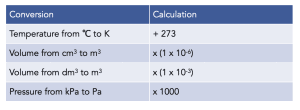
Step by Step Example
Practice Question: A gas occupies a volume of 1250cm3 at a temperature of 45℃ and a pressure of 105kPa. The gas has a mass of 2.4g. The gas constant (R) = 8.31 J K-1mol-1 . What is the relative molecular mass of the gas?
1. First write down all the values that you are given as written in the question.
Volume in cm3 = 1250cm3
Temperature in ℃ = 45℃
Pressure in kPa = 105kPa
Mass in g = 2.4g
R = 8.31 J K-1mol-1
2. Next convert all the values so that they are in the same units as the units used in the ideal gas equation.
Volume in m3 (V) = 1250 x (1 x 10-6) = 1.25 x 10-3
Temperature in K (T) = 45 + 273 = 318
Pressure in Pa (p) = 105 x 1000 = 105000 Pa
Mass in g = 2.4g
R = 8.31 J K-1mol-1
3. Then rearrange the ideal gas equation based on the values that are known.
pV = nRT
n = pV/RT
4. Finally enter the values into the equation
n = (105000 x (1.25 x 10-3)) ÷ (8.31 x 318) = 0.0497 moles (to 3 significant figures)
5. To calculate relative molecular mass use the equation: Relative molecular mass of gas = mass ÷ number of moles
Relative molecular mass of gas = mass ÷ number of moles = 2.4 ÷ 0.0497 = 48.3





Still got a question? Leave a comment
Leave a comment