Electrode Potentials and Electrochemical Cells - Half Cells and Full Cells (A-Level Chemistry)
Half Cells and Full Cells
Half Cells
Key Terms
A reduction is the gain of electrons.
An oxidation is the loss of electrons.
A reaction in which a reduction and an oxidation occur simultaneously is a redox reaction.
The oxidation state of an atom or ion is a measure of how oxidised or reduced it is. An oxidation reaction involves an increase in oxidation state. A reduction reaction involves a decrease in oxidation state.
Redox Couples
A redox couple is the combination of two forms of the same chemical species separated by the loss or gain of electrons so that they have two different oxidation states.
For example copper metal and copper (II) ions are a redox couple linked by the transfer of two electrons as shown in the half-equation:
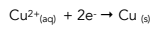
Redox Equilibrium
When we dip a metal into a solution of its ions a dynamic equilibrium or redox equilibrium gets established, where the rate of electron loss equals the rate of electron gain.
A copper / copper sulfate equilibrium can be set up in this way. Dipping a rod of copper into a solution of copper sulfate establishes this equilibrium: ![]()
This setup, where a rod of metal is dipped into a solution of its own ions to establish a redox equilibrium, is called a half cell, or an electrode.
The position of equilibrium will vary for different redox couples. The more easily reduced a metal, the further to the left the position of equilibrium will lie.
At equilibrium, the electrons on the metal strip set up a potential between the metal and the ions in solution.
This potential is an indication of how easily the metal loses electrons. The greater the tendancy for the metal to lose electrons, the greater the potential.
We cannot measure the potential of a single half cell, we can only measure the potential difference between two different half cells.
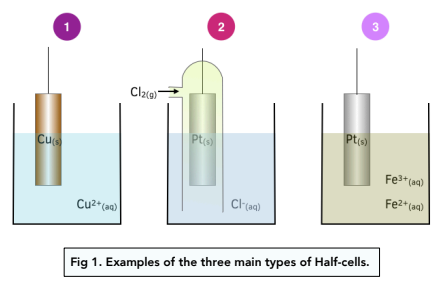
Types of Half Cells
There are there different types of half cells:
1. Metal/metal ion– This is the type of half cell we have discussed until now, where a metal rod is dipped into a solution of one of its ions.
2. Non-metal/non-metal ion – A platinum or graphite electrode is dipped into the non-metal ion solution. The non-metal gaseous element can then be bubbled over the electrode. The electrode is in contact with both the element and the aqueous ions so that the redox equilibrium gets established on its surface.
1. Ion/ion – The half cells contains a solution of two different ions of the same element, like for example Fe+2 (aq) and Fe+3 (aq). A platinum or graphite rod os used as the electrode.
Full (Electrochemical) Cells
Structure of an Electrochemical Cell
An electrochemical cell is formed when two half cells, consisting of different metals, or electrodes, in solutions of their ions, are connected with a wire.
A salt bridge completes the electrical circuit.
A salt bridge is typically a piece of filter paper soaked in a salt solution, often potassium nitrate (KNO3). It allows ions to flow.
Example: Zinc/Copper cell
The diagram below shows an example of an electrochemical cell.
The potential difference, or voltage, between the two half cells can be measured.
The zinc electrode has a greater tendancy to lose electrons than copper. The metal which is most easily oxidised is always placed on the left hand side of an electrochemical cell.
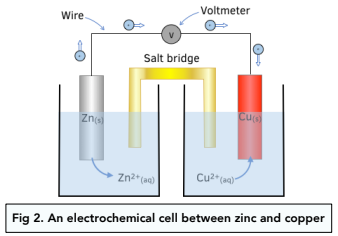
The wire connecting both half-cells, is also connected to a voltmeter. The voltmeter needs to have a very high resistance to prevent a current from flowing, so that the voltage can be measured.
If the voltmeter were removed, the following changes would occur:
1. Zinc metal is oxidised to form zinc ions. The zinc metal dissolves to form Zn²+ ions, thus reducing the size of the zinc metal and increasing the concentration of Zn²+ ions.
Oxidation reaction: Zn(s) → Zn²+(aq) + 2e-
2. A current sets up. Electrons would flow from the zinc, through the external wire, to the copper electrode.
3. Copper ions are reduced to form copper metal. Copper ions in the half cell on the right, will gain electrons to form copper metal. The size of the copper electrode increases as more copper metal is deposited, and the concentration of copper ions decreases.
Reduction reaction: Cu2+(aq) + 2e- → Cu
As time goes on, the half cell on the left will become increasingly positive, as more Zn²+ ions form, and the half cell on the right will become increasing negative, as less Cu²+ ions are present.
This will prevent electrons leaving the zinc electrode and moving to the copper half cell. Eventually the cell will stop working.
The salt bridge contains inert ions which move to balance the charges, and keep the cell working.
An electrode potential, also known as an electromotive force (EMF), is a measure of the voltage generated by a half-cell in an electrochemical cell. The electrode potential is a measure of the energy that is generated by the movement of ions between the half-cell and the solution in which it is immersed. It is a crucial concept in A-Level Chemistry, as it provides insight into the behavior of electrons in chemical reactions and allows us to understand the energy transfer that occurs during these reactions.
A half-cell is a single electrode in an electrochemical cell. It is composed of a metal and its ions in a solution. The electrode potential of a half-cell is determined by the energy required to move ions from the half-cell to the solution, and vice versa. By measuring the electrode potential of multiple half-cells, we can determine the energy difference between different electrode reactions and the overall energy balance of an electrochemical cell.
An electrochemical cell is a system that consists of two half-cells, each with a different electrode potential, that are connected by a salt bridge. The salt bridge allows ions to flow freely between the two half-cells, which creates a complete circuit and allows electrons to flow from one half-cell to the other. When an electrochemical cell is connected to an external circuit, the movement of electrons generates a voltage across the circuit, which can be used to perform work or to produce electrical energy.
A half-cell is a single electrode in an electrochemical cell, while a full cell is a complete electrochemical cell that consists of two half-cells connected by a salt bridge. The electrode potential of a half-cell is determined by the energy required to move ions from the half-cell to the solution, and vice versa. The overall voltage of an electrochemical cell, or full cell, is determined by the difference in electrode potential between the two half-cells.
The electrode potential of a half-cell determines the energy required to move ions from the half-cell to the solution, and vice versa. When two half-cells with different electrode potentials are connected in an electrochemical cell, the difference in electrode potential creates an overall voltage across the cell. The higher the difference in electrode potential between the two half-cells, the greater the overall voltage of the electrochemical cell.
The study of electrode potentials and electrochemical cells is important in A-Level Chemistry because it provides insight into the behavior of electrons in chemical reactions and the energy transfer that occurs during these reactions. This information is crucial for understanding the underlying mechanisms of electrochemical processes, such as corrosion, battery technology, and electroplating. Additionally, the knowledge of electrode potentials and electrochemical cells can be used to design new and more efficient electrochemical systems, such as fuel cells, solar cells, and electrochemical reactors.





Still got a question? Leave a comment
Leave a comment