Aromatic Chemistry - Reactivity of Substituted Benzene (A-Level Chemistry)
Reactivity of Substituted Benzene
Effects of Substituents on Reactivity
Electrophilic Substitution
Just like unsubstituted benzene, benzene rings with a functional group added to them also undergo electrophilic substitution but with some changes. The presence of a substituent group will affect:
- Position of electrophilic substitution – In the electrophilic substitution of benzene, it does not matter which carbon atom the substitution takes place at because all are the same. The presence of the substituent group will direct the electrophile towards specific positions.
- Reactivity – The presence of a substituent will also affect how reactive the benzene ring is, either increasing or decreasing it with respect to unsubstituted benzene.
The effects of the substituent group depends on whether it is an electron-donating or an electron-withdrawing group.
Electron-donating groups
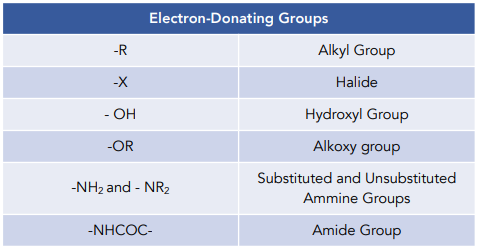
- Electron donating groups activate the benzene ring making it react faster. Electron donating groups have electron orbitals that overlap with the delocalised pi bonding system of benzene. As a result they increase the electron density of benzene making it more susceptible to attack by electrophiles.
- Electron donating groups direct incoming electrophiles to positions 2, 4 and 6. They do so by increasing electron density specifically at these positions.
Electron-withdrawing groups
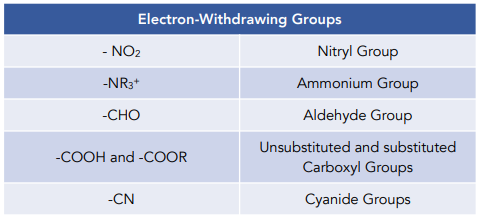
- Electron withdrawing groups deactivate the benzene ring making it react more slowly. Electron withdrawing groups do not have electron orbitals that overlap with the delocalised pi bonding system of benzene. They withdraw electron density from the benzene ring instead, making it less susceptible to attack by electrophiles.
- Electron withdrawing groups direct incoming electrophiles to position 3 and 5. They do so by withdrawing electron density specifically from positions 2, 4 and 6, making it less likely for electrophiles to react at these positions.
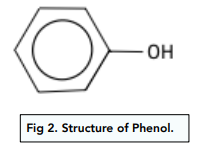
Example: Phenol
Phenol is an aromatic compound with formula C₆H₅OH consisting of a benzene ring with a -OH groups attached to it. -OH groups are electron-donating. Therefore, phenol is more reactive than benzene.
As a result, the reaction conditions needed for phenol to react are milder than for benzene. This can be seen for example in bromination and nitration.
Bromination
- Phenol reacts with aqueous bromine. Benzene is only able to react with bromine if it is in its pure gaseous form.
- Bromination of phenol takes place at room temperature. For benzene to undergo bromination, it needs to be heated and mixed with a halogen carrier catalyst.
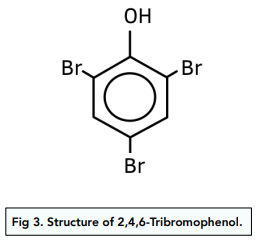
- Bromination of phenol takes place at multiple positions. The product of bromination of phenol is a white precipitate of 2,4,6- tribromophenol. In benzene only one bromine atom will get substituted to form bromobenzene.
Nitration
- Phenol can react with concentrated nitric acid at room temperature. Benzene only reacts with concentrated nitric acid if heated up to 55ºC and mixed with sulphuric acid.
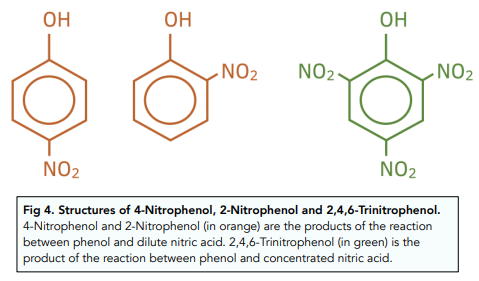
- Phenol undergoes nitration by concentrated nitric acid at various positions. The product of nitration is 2,4,6 nitrophenol. Nitration of benzene only results in one nitryl group being substituted.
- Phenol reacts with dilute nitric acid. When reacting with dilute nitric acid, only one nitric group gets substituted so that a mixture of 2- nitrophenol and 4-nitrophenol gets produced.
More on Phenol
Naming Phenols
Phenols are not named as substituted benzene compounds and instead are all given the sufix -phenol.
The carbon atom to which the -OH group is attached to is given number 1 and and all other functional groups on the benzene ring are numbered in such way the the lowest numbers are given.
Physical Properties
- Phenol is solid at room temperature. This is because hydrogen bonds can form between -OH groups.
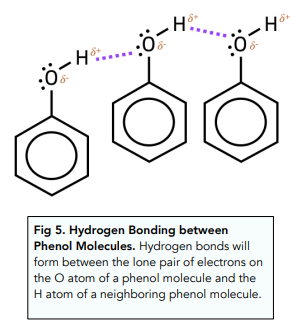
- Phenol is slightly soluble in water. Although phenol can form hydrogen bonds with water, the large non polar benzene ring disrupts the hydrogen bonding system so that phenol is unable to dissolve fully.
Phenol as a Base
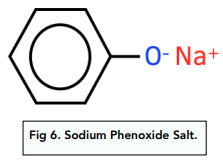
- Phenols dissociate in solution. They dissociate partially in solution to give H⁺ ions and phenoxide ions. This makes phenols behave like weak acids.
- Phenols are more acidic than water and other alcohols. This is because the phenoxide ion is more stable. As the lone pair of electrons on the oxygen atom becomes delocalised into the pi bonding system on the benzene ring, the negative charge on the phenoxide ion becomes spread out reducing its charge density. As a result, H⁺ ion are less strongly attracted to the phenoxide ions and less likely to reform undissociated phenol molecules.
- Phenols reacts with bases and metals to give phenoxide salts. It will react with bases such as sodium hydroxide in a neutralisation reaction to give a salt and water and with metals such as sodium to give a salt and hydrogen gas. It is not acidic enough to react with metal carbonates and form carbon dioxide.
- Just like alcohols, the -OH group in phenols can react with carboxylic acids to form an ester. For example, salicylic acid (a derivative of phenol) can be made to react with ethanoic anhydride at 50ºC and in the presence of a few drops of phosphoric acid, to make aspirin.
Formation of diazonium dyes
A diazonium dye is a colored compound that forms from the addition of a diazonium ion into the benzene ring of phenol or another aryl compound.
The structure of diazonium ion looks as follows:
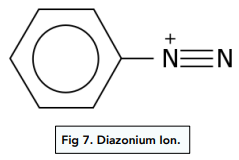
Diazonium dyes are characterized by the -N=N- group that acts as a bridge between the ion and the aryl compound. The delocalised pi bonding system extends between both benzene ring through this -N=N- group, which makes the diazonium dye very stable.
The colour of the dye will depend on the type of aryl compound used to make it. For example, the dye made from phenol is orange and it is synthesized in two steps:
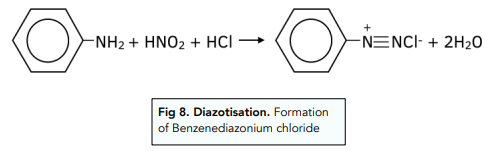
1. Formation of a diazonium salt. Phenylamine is a benzene ring with an amine side chain. Phenylamine reacts with nitric(III) acid (HNO₂) to from a diazonium salt. The nitric(III) is made in situ by reacting sodium nitrate(III) with dilute hydrochloric acid. The resulting salt is a benzenediazonium chloride. The reaction has to take place at 10ºC because otherwise the unstable diazonium salt would decompose into nitrogen gas.
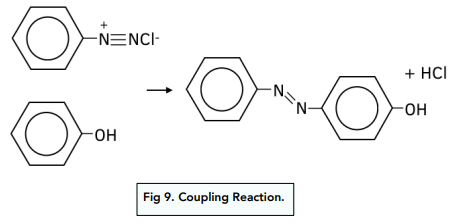
2. The diazonium salt reacts with phenol. The diazonium ion acts an electrophile and is substituted at position 4 of the benzene ring of phenol. The product of this coupling reaction is an orange diazonium dye and it forms immediately after both reagents are mixed.
Aromatic chemistry is the study of organic compounds that contain a cyclic, conjugated system of double bonds, such as benzene.
A substituted benzene is a benzene ring in which one or more of the hydrogen atoms have been replaced by other functional groups.
The presence of a substituent can affect the reactivity of a benzene ring by either increasing or decreasing its electron density, which in turn affects its ability to undergo electrophilic or nucleophilic substitution reactions.
An electron-donating group is a functional group that donates electrons to the benzene ring, thereby increasing its electron density. Examples of electron-donating groups include -OH, -NH2, and -CH3.
An electron-donating group increases the electron density of the benzene ring, making it more nucleophilic and less electrophilic. This makes it more likely to undergo reactions such as nucleophilic substitution.
An electron-withdrawing group is a functional group that withdraws electrons from the benzene ring, thereby decreasing its electron density. Examples of electron-withdrawing groups include -NO2, -COOH, and -CN.
An electron-withdrawing group decreases the electron density of the benzene ring, making it more electrophilic and less nucleophilic. This makes it more likely to undergo reactions such as electrophilic substitution.
The presence of multiple substituents can have a complex effect on the reactivity of a benzene ring. The nature, position, and number of the substituents can all influence the reactivity of the ring.
A meta-directing group is a functional group that directs electrophilic substitution to the meta-position of a substituted benzene ring. Examples of meta-directing groups include -NO2 and -COOH.
An ortho/para-directing group is a functional group that directs electrophilic substitution to the ortho or para position of a substituted benzene ring. Examples of ortho/para-directing groups include -OH, -NH2, and -CH3.





Still got a question? Leave a comment
Leave a comment