Thermodynamic - Lattice Enthalpies (A-Level Chemistry)
Lattice Enthalpies
Lattice Enthalpy
Key Terms
Enthalpy change is the change in heat energy of a substance at constant pressure. The symbol for enthalpy change is ΔH. If the enthalpy change is measured under standard conditions of temperature and pressure e say it is a standard enthalpy change and the symbol for it is ΔH°.
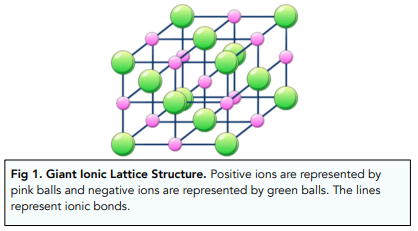
Enthalpy of lattice formation is the standard enthalpy change when one mole of solid ionic crystal lattice is formed from its gaseous ions. Enthalpy of lattice formation is always exothermic, so will have a negative value,

Enthalpy of lattice dissociation is the standard enthalpy change when one mole of solid ionic crystal lattice is fully dissociated into its gaseous ions. Enthalpy of lattice dissociation is always endothermic, so will have a positive value,

Lattice enthalpies are used as a measure of the strength of ionic bonding: the greater the value of the lattice enthalpy of an ionic compound, the stronger its ionic lattice.





Still got a question? Leave a comment
Leave a comment