Properties of Period 3 Elements - Reactivity of Period 3 Elements (A-Level Chemistry)
Reactivity of Period 3 Elements
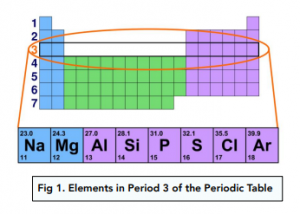
Period 3 Elements
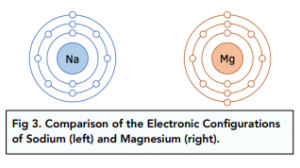
Period 3 elements are in the third row of the periodic table. Each element has 3 shells overall.
The period number tells you the number of shells – e.g. all Period 3 elements have 3 shells.
The group number tells you the number of electrons in the outermost shell – e.g. Sodium (Group 1) has 1 outer electron in the third outer shell.
Reactions with Water
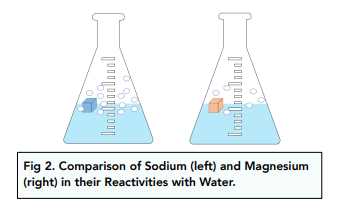
The only metal elements in period 3 that will react with cold water are sodium and magnesium.
Sodium + Water
Sodium reacts with water in a rapid and violent reaction. The sodium will fizz and float on the surface of the water as it reacts. It is an exothermic reaction.
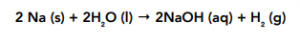
The resulting solution is a very strongly alkaline solution of sodium hydroxide, this has a pH of about 13-14.
Magnesium + Water
Magnesium reacts less violently and less rapidly than sodium. It is a very slow reaction at room temperature.
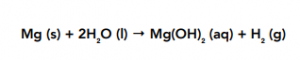
The resulting solution of this reaction is a weak alkaline – not as strongly alkaline as the one produced by the reaction between sodium and water. This is because magnesium is less soluble in water in water than sodium.
This solution has a pH of about 10.
Why is Magnesium less reactive than Sodium?
Magnesium has a smaller atomic radius and higher charge compared with sodium. This results in a greater electrostatic attraction holding the magnesium lattice together. This is why magnesium is less reactive than sodium. The reaction has a much higher activation energy
When magnesium is heated with steam, the following, very exothermic reaction occurs:
Reactions with Oxygen
All Period 3 elements can form oxides when they react with oxygen.
These are exothermic reactions.
Sodium + Oxygen
Sodium will react directly with oxygen and burns brightly to give a characteristic bright yellow flame. Sodium oxide is formed.
Magnesium + Oxygen
Magnesium ribbon burns readily in oxygen to produce magnesium oxide. The reactions gives a bright white flame and leaves a white powder of magnesium oxide.
Aluminium + Oxygen
Aluminium will react quite slowly with oxygen unless it is in a powdered form. It will burn brightly to produce a white powder of aluminium oxide.
Silicon + Oxygen
Silicon will react with oxygen quite slowly to produce silicon dioxide. It must be heated strongly in oxygen.
Phosphorus
Phosphorus has two allotropes. These are different physical forms of the same element.
Red phosphorus must be heated before it reacts with oxygen.
White phosphorus will react spontaneously with oxygen to produce a white smoke of phosphorus pentoxide.
Sulfur
Sulfur reacts with oxygen to form sulfur dioxide. Unlike the other elements in period 3, it is not fully oxidised to its highest oxidation state of +6 – it only reaches an oxidation state of +4.
A catalyst and a high temperature is required to reach +6 and form SO₃.
FAQs
The properties of Period 3 elements are the characteristics that define their behavior in chemical reactions and their physical properties. These properties include atomic number, electron configuration, reactivity, melting point, boiling point, density, and more. Understanding the properties of Period 3 elements is crucial in A-Level Chemistry, as they form the basis for understanding more complex chemical reactions and processes.
The reactivity of Period 3 elements refers to their tendency to participate in chemical reactions and form compounds with other elements. Reactivity is influenced by several factors, including the electron configuration of the element, the strength of its chemical bonds, and the energy required to break these bonds. In general, Period 3 elements are less reactive than elements in earlier periods and become increasingly less reactive as one moves from left to right across the periodic table.
The electron configuration of an element plays a major role in determining its reactivity in A-Level Chemistry. Elements in Period 3 have electron configurations that differ in the number and arrangement of electrons in their outermost energy level, or valence shell. These differences can affect the strength of chemical bonds and the energy required to break them, which in turn influences the reactivity of the element. For example, elements with more electrons in their valence shell are more likely to participate in chemical reactions and form compounds with other elements.
The position of a Period 3 element on the periodic table affects its reactivity in A-Level Chemistry because it determines the number of electrons in the element’s outermost energy level, or valence shell. Elements in the left-hand side of the periodic table have fewer electrons in their valence shell and are generally more reactive than elements on the right-hand side, which have more electrons in their valence shell and are generally less reactive. This trend is known as the reactivity series and is a useful tool for predicting the behavior of elements in chemical reactions.
The reactivity series is a list of elements in order of their reactivity in A-Level Chemistry. The reactivity series is useful for predicting the behavior of elements in chemical reactions, as it shows the relative reactivity of different elements. Elements that are high in the reactivity series are more reactive and are more likely to participate in chemical reactions, while elements that are low in the reactivity series are less reactive and are less likely to participate in chemical reactions.
The study of the reactivity of Period 3 elements is important in A-Level Chemistry because it provides insight into the behavior of elements in chemical reactions and the underlying mechanisms of these reactions. Understanding the reactivity of Period 3 elements is crucial for predicting the outcome of chemical reactions and for designing new and more efficient chemical processes. Additionally, the knowledge of the reactivity of Period 3 elements is essential for understanding the properties and behavior of more complex chemical systems, such as catalysts, batteries, and fuels.





Still got a question? Leave a comment
Leave a comment