Amount of Substance - Measuring Relative Masses (A-Level Chemistry)
Measuring Relative Masses
Relative Masses
Definitions
A unified atomic mass unit is a unit of mass commonly used in chemistry which corresponds to one twelfth of the mass of a neutral carbon-12 atom. Relative masses are measured in terms of unified atomic mass units.
The relative atomic mass (Ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of carbon-12. Carbon is used as the standard to which the relative masses of other elements are compared and it has a relative atomic mass of 12. You can find the relative atomic mass of an element on a periodic table by looking at the number directly above the element symbol. For example the relative atomic mass of Copper (Cu) is 29.
The relative molecular mass (Mr) is the average mass of a molecule relative to one twelfth of the mass of an atom of carbon-12. It is the sum of the relative atomic masses of each atom within the molecule.
The relative isotopic mass is the mass of an atom of an element relative to one twelfth of the mass of an atom of carbon-12.
The relative formula mass is the relative mass used for giant covalent compounds or ionic compounds. An example of a giant covalent or ionic compound is Mg(OH)2.
We use the same method to calculate the relative formula mass as we would use for the relative molecular mass. We will look at this method below.
Calculating RAM
We learnt earlier how we can calculate RAM using the relative abundance of isotopes of an element.
Practice Question: Work out the RAM of chlorine, using the information in the table below.
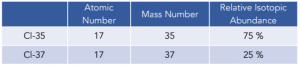
1. Multiply the relative isotopic abundance (%) by the mass of each isotope, and add them to each other.
(35 x 75) + (37 x 25) = 3550
2. Divide by 100 and do a sense-check.
3550 / 100 = 35.5
We can do a sense-check, to make sure that the value seems right. 35.5 is in between 35 and 37, as we would expect, and it has a closer mass to Cl-35, which is the more abundant isotope. This seems fine!
Calculating RMM and RFM
To calculate the relative molecular mass (RMM) or the relative formula mass (RFM) we have to add the relative atomic masses of each atom within the molecule. Let us work through calculating the RMM / RFM of a molecule of water.
Practice Question: Work out the RMM of water.
1. First of all write out the formula of the molecule
Water has a formula of H2O.
2. Next write down which elements are present and their respective relative atomic masses.
The elements that are present are:
- 2 hydrogen atoms, which each have a relative atomic mass of 1
- 1 oxygen atom, which has a relative atomic mass of 16
3. Then add up all the relative atomic masses of all the elements present in the molecule.
Therefore the relative molecular mass of water is: (1 x 2) + 16 = 18
The amount of substance, also known as the amount of matter, refers to the quantity of a chemical substance present in a sample. It is a basic concept in chemistry that helps to determine the relative masses of different substances.
The amount of substance can be measured using various units such as moles, grams, or particles. The most common unit used in chemistry is the mole, which represents a specific number of particles (usually atoms or molecules) in a sample.
The amount of substance is important in A-Level Chemistry because it helps to determine the relative masses of different substances. This information is crucial for understanding chemical reactions, as the number of particles present in a reaction can affect the outcome of the reaction.
The mole is directly related to the relative masses of substances because it represents a specific number of particles in a sample. By using the mole as a unit of measurement, it becomes possible to compare the relative masses of different substances and determine the amount of substance needed for a reaction to occur.
The Avogadro constant is the number of particles in one mole of a substance. It is important in A-Level Chemistry because it helps to convert between the mole and other units of measurement, such as grams. This constant is a fundamental concept in chemistry, as it helps to determine the number of particles present in a sample, which is necessary for understanding chemical reactions.
To convert between moles and grams in A-Level Chemistry, you need to use the formula:
mass (in grams) = moles x molar mass
The molar mass is the mass of one mole of a substance and can be found by adding up the atomic masses of all the atoms in the formula of the substance.
“Relative Atomic Mass” (RAM) is the average mass of an atom of an element, compared to the mass of an atom of carbon-12. It is measured using a mass spectrometer, which separates atoms based on their mass-to-charge ratio and measures their mass.
“Relative Molecular Mass” (RMM) is the average mass of a molecule, compared to the mass of an atom of carbon-12. It is calculated by adding up the RAMs of all the atoms in a molecule. RMM can also be measured using a mass spectrometer, by ionizing the molecule and measuring its mass-to-charge ratio.
“Percentage Yield” is a measure of the efficiency of a chemical reaction, calculated as the actual yield of a product divided by the theoretical yield, multiplied by 100%. Theoretical yield is the amount of product that should be produced based on stoichiometric calculations, while actual yield is the amount of product obtained in a real experiment. Percentage yield can be affected by factors such as impurities in the starting materials, incomplete reactions, and side reactions.
“Empirical Formula” is the simplest whole number ratio of atoms in a compound. It can be calculated from the percentage composition of the elements in the compound, by dividing the percentage of each element by its RAM and converting the resulting values to a whole number ratio. The empirical formula can then be used to calculate the molecular formula, which gives the actual number of atoms of each element in the compound.





Still got a question? Leave a comment
Leave a comment