Thermodynamic - Calculations involving Free Energy (A-Level Chemistry)
Calculations involving Free Energy
Feasibility and Temperature
Temperature Change and Reaction Spontaneity
For a reaction to be feasible, ΔG must be negative. Temperature can influence the feasibility of a reaction.
In order to predict the feasibility of the reaction we have to look at the two terms that make up the equation for ΔG.
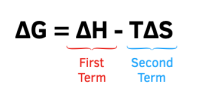
If a reaction is exothermic (ΔH is negative) and it has a:
- Positive entropy change (ΔS is positive) – the reaction will always be feasible – ΔG is always negative.
- Negative entropy change (ΔS negative) – the feasibility of the reaction will depend on temperature. The lower the temperature, the more likely it is for the positive value of the second term to be greater than the negative value of the first term and for ΔG to be negative.
If a reaction is endothermic (ΔH is positive) and it has a:
- Positive entropy change (ΔS is positive) – the feasibility of the reaction will depend on temperature. The higher the temperature, the more likely it is for the negative value of the second term to be greater than the positive value of the first term and for ΔG to be negative.
- Negative entropy change (ΔS negative) – the reaction will never be feasible – ΔG is always positive.
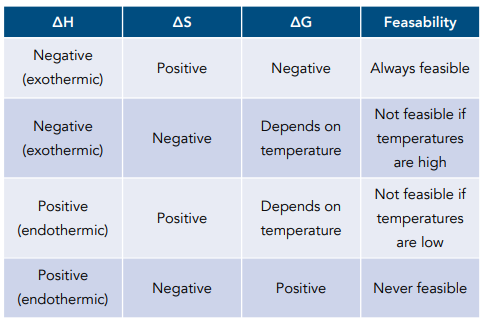
Calculating the Temperature at which a Reaction becomes Feasible
When ΔG is negative or zero, a reaction is feasible.
When ΔG is zero, this is the temperature when a reaction is just feasible.
Rearranging the equation ΔG = ΔH – TΔS allows you to find the temperature at which a reaction just becomes feasible.
When ΔG = 0:

Example: Find the temperature at which this reaction just becomes feasible.
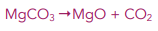
ΔG = 0 therefore T = ΔH / ΔS
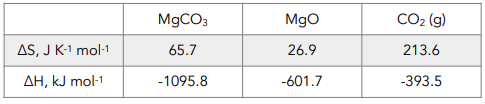
1. Calculate enthalpy change. This is done by subtracting the total enthalpy of the reactants from the total enthalpy of the products.
ΔH = ΣΔH (products) – ΣΔH (reactants)
= [(-601.7) + (-393.5)] – (-1095.8)
= +100.6 kJ mol-1
2. Calculate entropy change. This refers specifically to entropy change of the system, which can be calculated by subtracting the total enthalpy of the reactants from the total enthalpy of the products.
ΔS = ΣΔS (products) – ΣΔS (reactants)
= [(26.9) + ( 213.6)] – (65.7)
= +174.8 J K-1 mol-1
3. Calculate the temperature at which the reaction becomes feasible. Simply substitute the values for enthalpy change and entropy change into the equation.
T = ΔH / ΔS
T = 100.6 / 174.8 x 1000
T = 576 K
Gibbs Free Energy and Equilibrium Constants
Relationship between Equilibrium Constants and ΔG
The equilibrium constant of a reversible reaction is the ratio of the concentration of products to the concentration of the reactants at equilibrium.
Reactions with a negative ΔG will have large equilibrium constants with values greater than one.
Reactions with positive ΔG have small equilibrium constants with values less than 1.
This relationship is illustrated by the following equation:
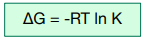
Where:
ΔG = Change in Gibbs free energy, kJ mol-1
R = Gas constant, 8.31 J K-1 mol-1
T = Temperature, Kelvin
ln K = Natural logarithm of the equilibrium constant
Calculating Equilibrium Constants from ΔG
The equilibrium constant of a reaction can be calculated from ΔG by rearranging the previous equation so that:
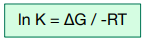
Example: The reaction between hydrogen and chlorine gases to form hydrogen iodide is a reversible reaction with equation H2 (g) + I2 (g)→ 2HI (g) and ΔG = -24287 J mol-1. What would the equilibrium constant for the reaction at 273K be?
1. Calculate the value for K. The inverse of a natural logarithm is the exponential of e.
ln K = ΔG / -RT
ln K = -24287 J mol-1 / [- 8.31 J K-1 mol-1 x 273 K] = 3.8304
K = e3.8304 = 46
2. Calculate the units of K. This is done by finding out the equation for the equilibrium constant and substituting values for their units.
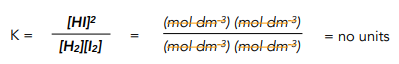
Free energy, also known as Gibbs free energy, is a thermodynamic quantity that represents the amount of energy available to do work. It takes into account the temperature, entropy, and enthalpy of a system and can be used to determine whether a chemical reaction is spontaneous or not.
Free energy is calculated using the equation ΔG = ΔH – TΔS, where ΔG is the change in free energy, ΔH is the change in enthalpy, T is the temperature in Kelvin, and ΔS is the change in entropy.
A negative free energy change indicates that a reaction is spontaneous and will proceed as written, releasing energy in the form of heat or light.
A positive free energy change indicates that a reaction is not spontaneous and will require an input of energy in order to proceed.
Yes, the value of free energy can change with temperature. As temperature increases, the entropy of a system also increases, which can cause the value of ΔG to change.
The concept of free energy is an important topic in A-Level Chemistry as it helps students understand the behavior of chemical reactions and the energy changes that occur during those reactions. By understanding free energy, students can predict whether a reaction will occur spontaneously or not and determine the conditions under which a reaction is thermodynamically favorable.
Free energy calculations can be used in a variety of real-world applications, including the design of energy-efficient systems, the prediction of reaction rates and yields, and the optimization of industrial processes. The understanding of free energy is also crucial in the development of new technologies and materials, such as alternative energy sources and superconductors.






Still got a question? Leave a comment
Leave a comment