Halogenoalkanes - Reactivity of Halogenoalkanes (A-Level Chemistry)
Reactivity of Halogenoalkanes
Nucleophilic Substitution
Key Terms
Substitution reactions – A reaction in which a functional group in a compound is replaced by (or is substituted by) another.
Nucleophile – An atom or compound with a negative or partial negative charge which is able to form a covalent bond by donating a lone pair of electrons.
Carbocation – Hydrocarbon molecule in which one of its carbon atoms is only bonded to three other atoms and has a positive charge.
Nucleophilic Substitution in Halogenoalkanes
In a nucleophilic substitution, a nucleophile provides a pair of electrons to the partially positively charged carbon in the halogenoalkanes. The Chalogen bond breaks heterolitically, with both electrons being taken by the halide ion. A new bond forms between the carbon atom and the nucleophile. The halogen has been replaced by another atom group.
The three nucleophilic substitution reactions which you need to know are the reactions with OH- , CN- and NH3
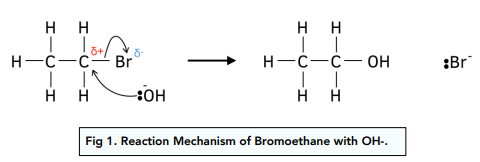
Nucleophilic substitution: OH Groups
- Nucleophilic substitution by OH groups forms alcohols. OH groups in aqueous alkalis such as potassium and sodium hydroxides are very nucleophilic. There is a lone pair of electrons on the O atom which attacks the positively charged C atom. The halogen atom is replaced by the OH group to form an alcohol. This is a hydrolysis reaction.
- OH groups can also come from water. Water can also act as a weak nucleophile to produce an alcohol but at a much slower rate.
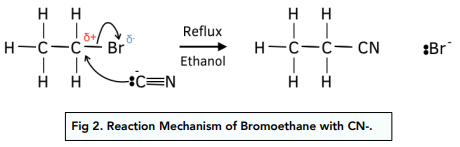
Nucleophilic substitution: CN Groups
- Halogenoalkanes react with CN- ions to form nitriles. CN groups in potassium or sodium cyanide are weakly nucleophilic. The lone pair on the CN- ion will attack the partially charged carbon atom so that the halogen is replaced by a CN group and a nitrile forms.
- The length of the carbon chain is increased in this reaction. The CN group adds another carbon atom to the existing halogenoalkane.
- The reaction must take place under reflux conditions and in ethanol. Water in an aqueous solution would act as a nucleophile and undergo nucleophilic substitution with the halogenoalkane, forming an alcohol instead.
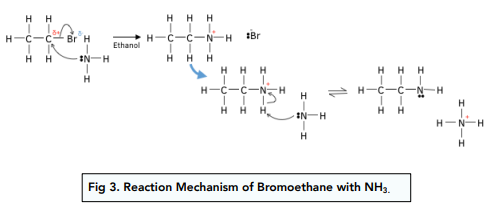
Nucleophilic substitution: NH3
- Halogenoalkanes react with ammonia to form amines. First, the lone pair on NH3 attacks the partially charged carbon so that the a C-N bond replaces the C-halogen bond. The NH3 displaces the halogen and has a positive charge. The leaving halide ion or another ammonia molecule in solution then picks up a H+ from the NH3 by heterolytic fission and leaving an amine group.
- For the reaction to occur, it must take must take place in ethanol and in excess ammonia. Again, if the reaction occurred in aqueous solution, water would act as a nucleophile and an alcohol would form instead. Amines are also strong nucleophiles so in the absence of excess ammonia, they would undergo nucleophilic substitution with the halogenoalkane.
- Ammonium salts are formed as a by product. The extra H+ in NH3 is picked up by the leaving halide ion to form an acid (i.e.: HCl, HBr). This reacts with the excess ammonia to form ammonium salts. If the extra H+ is picked up by another NH3 molecule instead, an ammonium ion (NH4+) forms, which along with the halide ions in solution can form ammonium salts.
Reaction Mechanisms of Nucleophilic Substitution
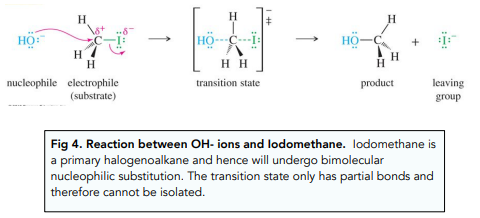
Nucleophilic Substitution: Primary Halogenoalkanes
The reaction between primary halogenoalkanes and a nucleophile happens in only one step: the C-halogen bond breaks at the same time as the C-Nucleophile bond forms.
This process is also known as bimolecular nucleophilic substitution (SN2) because the rate of the reaction depends on the concentration of both reactants: the halogenoalkane and the nucleophile.
It involves the transient formation of a high energy transition state which disappears very quickly and cannot be isolated.
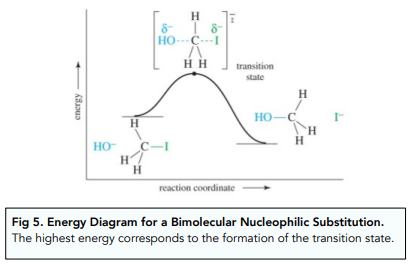
Nucleophilic Substitution: Tertiary Halogenoalkanes
The reaction between tertiary halogenoalkanes and a nucleophile happens in two steps.
First the C-halogen bond is broken by heterolytic fission to form a carbocation. The nucleophile rapidly attacks the charged carbocation to form a new C-nucleophile bond.
This process is also known as unimolecular nucleophilic substitution (SN1) because the rate of the reaction only depends on the concentration of one of the reactants: the halogenoalkane. This is because the first step of the reaction is the rate limiting step.
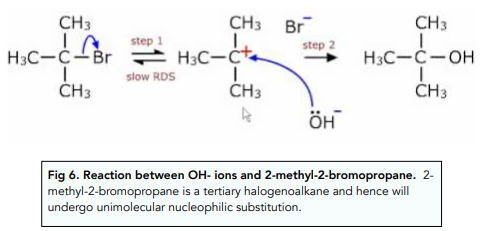
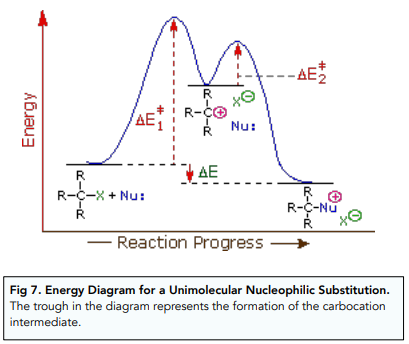
Nucleophilic Substitution: Secondary Halogenoalkanes
The reaction mechanism of nucleophilic substitution in secondary halogenoalkanes will be a mixture of the other two reaction mechanisms.
Elimination
Key Terms
Elimination reactions – a reaction in which an atom or a group of atoms in a compound is removed from a molecule.
Reaction Mechanism of Elimination
The conditions in which halogenoalkanes react with OH- ions, from potassium hydroxide, determines which type of reaction occurs.
If it occurs in aqueous conditions, a nucleophilic substitution occurs, resulting in the formation of an alcohol. However, when it occurs in ethanol under reflux conditions, an alkene is produced in an elimination reaction.
For example for the reaction of 2-chlorobutane with KOH in ethanol the mechanism is as follows:
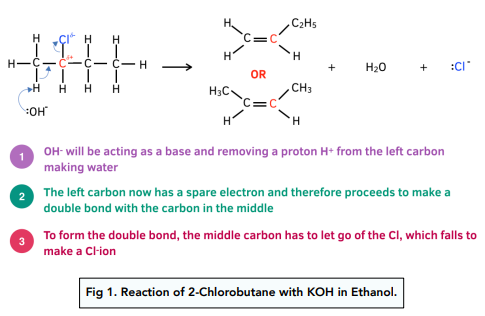
Worked example: 2-bromo-2-methylpentane reacts with potassium hydroxide dissolved in ethanol. Two possible isomers are formed. Draw the mechanism for both reactions and name each isomer.
Answer:
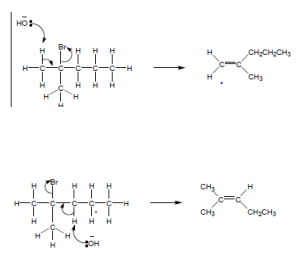
The isomer from the first reaction = 2-methylpent-1-ene.
The isomer from the second reaction = 2-methylpent-2-ene.
Halogenoalkanes are organic compounds composed of an alkane molecule and one or more halogens (e.g. fluorine, chlorine, bromine, or iodine).
The reactivity of halogenoalkanes depends on the type of halogen present. Generally, the reactivity decreases in the order: fluorine > chlorine > bromine > iodine.
Yes, halogenoalkanes can undergo substitution reactions, in which the halogen atom is replaced by another substituent.
The type of reaction that a halogenoalkane will undergo depends on the conditions in which it is reacted and the nature of the halogen atom. If the reaction occurs in the presence of an acidic catalyst, a substitution reaction is likely to occur. On the other hand, if the reaction occurs in the presence of a strong base, an elimination reaction is more likely.
Yes, halogenoalkanes can also undergo elimination reactions, in which two halogen atoms are eliminated from the molecule.
The difference between primary, secondary, and tertiary halogenoalkanes depends on the number of carbon atoms attached to the carbon that holds the halogen. In primary halogenoalkanes, only one carbon atom is attached to the halogen-containing carbon. In secondary halogenoalkanes, two carbon atoms are attached, and in tertiary halogenoalkanes, three carbon atoms are attached.
The reactivity of halogenoalkanes increases as the number of carbon atoms attached to the halogen-containing carbon increases. This means that primary halogenoalkanes are the least reactive, while tertiary halogenoalkanes are the most reactive.






Still got a question? Leave a comment
Leave a comment