Amines - Introduction to Amines (A-Level Chemistry)
Introduction to Amines
Amines
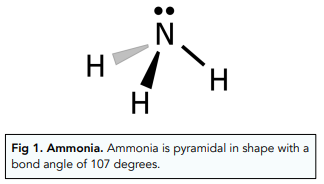
Amines are molecules with the functional group R-NH₂.
Amines are derived from ammonia (NH₃).
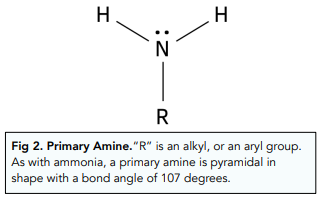
Primary Amines
If one of the H atoms from ammonia is replaced with an “R” group (either an alkyl or aryl group), a primary amine is formed.
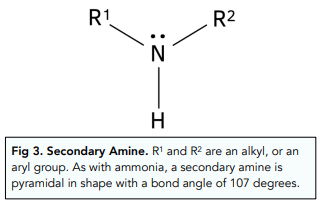
Secondary Amines
If two of the hydrogen atoms from ammonia are replaced with alkyl or aryl chains, a secondary amine is formed.
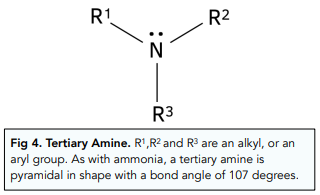
Tertiary Amines
In a tertiary amine, all three H atoms from the ammonia molecule are replaced with R groups.
Naming Amines
Naming Primary Amines
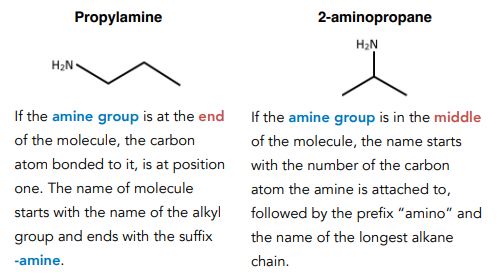
Naming Secondary Amines
Use the prefix N- to show that there is a smaller alkyl group attached to the N atom. Follow this with the name of the smaller alkyl group. Then use the name of the longest alkyl chain. End with the suffix -amine
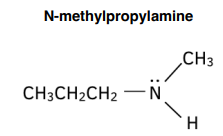
Naming Tertiary Amines
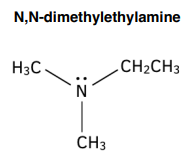
- Use the prefix N,N- to show that there are two smaller alkyl groups attached to the N atom.
- Follow this with the names of the smaller alkyl groups, in alphabetical order.
- Use the prefix di- if two of the alkyl groups are the same and tri- if all three are the same.
- Then add the name of the longest alkyl chain.
- End with the suffix -amine.
Worked Example: Name each of the amines given and identify whether they are primary, secondary or tertiary amines.
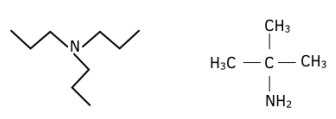
Answer: The first is N,N-tripropylamine; it is a tertiary amine. The second is a primary amine; it is called 2-methyl-propyl-2-amine.
Amines are a type of organic compound that contain nitrogen and hydrogen atoms. They are basic in nature and are classified based on the number of nitrogen atoms in their structure. Amines are a diverse class of compounds, with a wide range of applications, including their use as drugs, dyes, and disinfectants.
Amines have a basic nitrogen atom with one, two, or three alkyl or aryl groups attached to it. The nitrogen atom in an amine is bonded to one, two, or three hydrogen atoms or organic groups. The organic groups can be either alkyl or aryl groups.
There are three main types of Amines: Primary Amines, Secondary Amines, and Tertiary Amines. The classification of an Amine depends on the number of carbon atoms bonded to the nitrogen atom. Primary Amines have one carbon atom bonded to the nitrogen, Secondary Amines have two, and Tertiary Amines have three.
Amines are basic in nature and have a basicity that depends on their structure. The basicity of an Amine is determined by the number of alkyl or aryl groups bonded to the nitrogen atom. Primary Amines are the most basic, followed by Secondary Amines, and Tertiary Amines are the least basic.
Amines and Amides are similar in structure, but they differ in the functional group that is attached to the nitrogen atom. Amines have an NH2 group, while Amides have an NC=O group. This difference in functional group also leads to differences in properties and reactivity between the two compounds.
Amines are an important class of compounds in A-Level Chemistry and are studied in detail to understand their structure, properties, and reactions. They are also used as examples to illustrate the concept of basicity and the importance of functional groups in organic chemistry.
Amines have a wide range of real-life applications, including their use as drugs, dyes, and disinfectants. They are also used as intermediates in the synthesis of other chemicals, such as polymers and pharmaceuticals. Additionally, some Amines are used as food additives and as agricultural chemicals.





Still got a question? Leave a comment
Leave a comment