Introduction to Organic Chemistry - Naming and Representing Organic Compounds (A-Level Chemistry)
Naming and Representing Organic Compounds
Organic Compound Nomenclature
Key Terms
Organic compounds are compounds that make up the cells of living organisms and consist mainly of carbon atoms bonded to each other and to atoms of other elements by covalent bonds.
A functional group is the reactive part of an organic molecule.
Types of formulae
The empirical formula is the simplest ratio of elements in a molecule. For example, the empirical formula of glucose is CH2O. It actually contains 6 x C atoms, 12 x H atoms and 6 X O atoms.
The molecular formula is the actual number of atoms of each element present within the molecule. For example the molecular formula of glucose is C6H12O6.
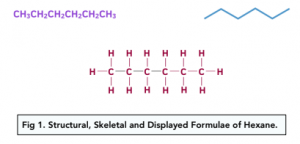
The displayed formula is the fully drawn out version of a molecule. It shows each of the bonds in full.
The structural formula is the molecule written with each carbon listed separate. For Hexane this would be: CH3CH2CH2CH2CH2CH3
The skeletal formula is a representation of organic compound in which the carbon chain is shown by lines with no carbons or hydrogens drawn. It is assumed there are carbon atoms where the bonds meet. It shows only a ‘skeleton’ with just functional groups drawn out. The angle of the C-C bonds in an unbranched chain should be about 109.5°.
Worked example: A hydrocarbon contains 240 g of carbon and 44 g of hydrogen.
a) Determine the empirical formula.
b) If the molecular mass is 142, determine its molecular formula.
c) Suggest it’s structural formula
d) Draw the skeletal formula.
Answer:
a) Step 1: Find the number of moles of each element
Moles of C = 240/12 = 20
Moles of H = 44/1 = 44
Step 2: Divide by the smallest number of moles to find the simplest ratio
C:H = 20:44 = 5:11
Empirical formula = C5H11
b) To find the molecular formula:
Step 1: Divide the molecular mass by the mass of the empirical formula.
Mass of empirical formula = (5 x 12)+ (11 x 1) = 71
142/71 = 2
Step 2: Multiply the empirical formula by the answer to give the molecular formula.
(C5H11) x 2 = C10H22
c) The structural formula is CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
d) ![]()
Naming Compounds
To name any compound there are some key rules to remember.
1. Determine the length of the longest carbon chain. This is key as it will give you the stem of the compound’s name.
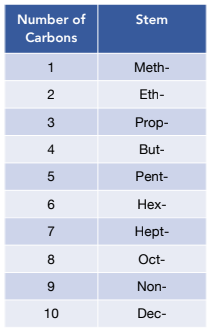
2. Find the functional groups. These are the reactive parts of the molecule. If there is only one functional group, it will be named as the suffix of the compound. These give us the prefix and suffix of the compound. The key functional groups you need to know are:
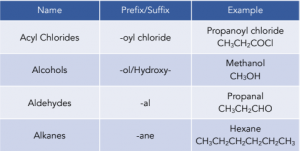
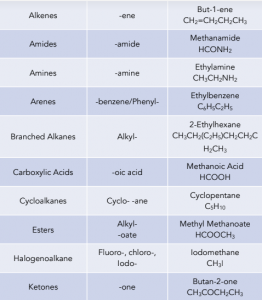
3. Give the longest chain of carbon numbers. Ensure that the carbon bonded to the functional group is given a number as small as possible.
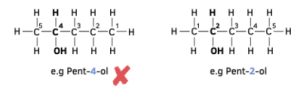
4. Determine the suffix. Include this number as part of the name in front of the suffix e.g. Butan-1-ol
5. Determine the prefixes. Other functional groups and carbon side- chains are included in the name as prefixes. These are written in alphabetical order and also require the number of the carbon they are attached to. e.g. 1 iodomethanol – ICH2OH
6. Adapt for any double or triple chains. When there are identical side-chains di- is used for 2, tri- for 3, and tetra- for 4.
Worked example: Give the name for the following molecule:
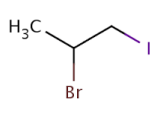
Answer: 2-bromo-1-iodopropane
Explanation: The longest carbon chain has three carbon atoms.
Therefore the prefix “prop” is used for this.
“Bromo” is written before “iodo” as the functional groups are written in alphabetical order.
Drawing Compounds
When drawing these compounds:
1. Draw the carbon chain. Draw out the carbon chain described in the stem – e.g. 6 Carbons for Hexanol (this could be in skeletal or displayed formulae).
2. Use the suffix to add functional groups. Add the primary functional group given by the suffix, in the position described by the number.
3. Add carbon sides and secondary functional groups. Next add the carbon side-chains and any secondary functional groups in the places described by the numbers.
4. Add in remaining hydrogens. Finally, add in the remaining hydrogens, ensuring each carbon forms only 4 bonds.
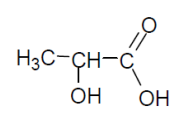
Worked example: Draw the structure of 2-hydroxypropanoic acid.
Answer:
The IUPAC system is the International Union of Pure and Applied Chemistry system for naming organic compounds. It is based on the structure of the compounds and provides a standardized naming system for organic compounds.
To name an alkane using the IUPAC system, you must first find the longest carbon chain in the compound. This chain is called the parent chain. Then, name the parent chain using Greek prefixes to indicate the number of carbons, and add the suffix -ane to indicate that it is an alkane.
To name an alkene using the IUPAC system, you must first find the longest carbon chain in the compound that contains the double bond. This chain is called the parent chain. Then, name the parent chain using Greek prefixes to indicate the number of carbons, and add the suffix -ene to indicate that it is an alkene.
To name an alcohol using the IUPAC system, you must first find the longest carbon chain in the compound that contains the hydroxyl group (-OH). This chain is called the parent chain. Then, name the parent chain using Greek prefixes to indicate the number of carbons, and add the suffix -ol to indicate that it is an alcohol.
To name a carboxylic acid using the IUPAC system, you must first find the longest carbon chain in the compound that contains the carboxyl group (-COOH). This chain is called the parent chain. Then, name the parent chain using Greek prefixes to indicate the number of carbons, and add the suffix -oic acid to indicate that it is a carboxylic acid.
To represent organic compounds using structural formulas, you can use line structures or bond-line structures. Line structures show the bonds between atoms as lines, while bond-line structures use abbreviated symbols to represent bonds and atoms. Both types of structures provide a visual representation of the arrangement of atoms in an organic compound.






Still got a question? Leave a comment
Leave a comment