Rate Equations - Temperature Changes and the Rate Constant (A-Level Chemistry)
Temperature Changes and the Rate Constant
Effects of temperature changes
Effects of changes in temperature on the reaction rate
Increasing the temperature means that a larger proportion of particles will have at least the minimum of the activation energy.
A greater proportion of particles have enough energy to react, i.e energy above the activation energy, therefore rate of reaction increases.
As you know from the Maxwell-Boltzmann distribution curve, the area to the right of the Ea line illustrates the number of particles with at least a minimum of the activation energy. This area is significantly greater at a higher temperature.
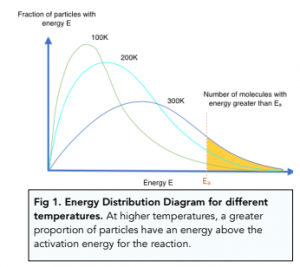
Effects of changes in temperature on the rate constant
The value of the rate constant k depends on temperature.
We know that if we increase the temperature, the rate of reaction will increase. We also know that when we increase the temperature, the values of [A] and [B] will not change. It follows, therefore, that k must change with temperature.
The Arrhenius Equation
The Arrhenius equation allows us to link temperature, T, the rate constant, k, and activation energy, Ea. It shows us how k varies with these factors.

Where:
k = rate constant (units depend on the rate equation)
A = the Arrhenius constant (units the same as k)
Ea = activation energy (J)
R = the gas constant (8.31JK−¹mol−¹)
T = temperature (K)
The e−Ea/RT part of the Arrhenius equation represents the fraction of molecules with energy greater than the activation energy.
The constant, A, is related to the number of collisions between reactant molecules.
Worked example: Calculate the rate constant at 50°C when the Arrhenius constant, A = 2.5 x 106 s-¹ and the activation energy Ea = 75 kJ mol-¹
Answer:
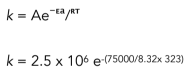
k = 1.89 x 10-6 s-¹
Logarithmic Form of the Arrhenius equation
The Arrhenius equation can be used to calculate the rate constant or the activation energy.
You need to take natural logs of the Arrhenius equation and then rearrange to get whatever term you are trying to work out on one side by itself.

Rearranging the equation:
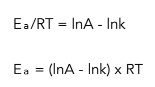
This equation can also be written as:
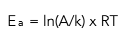
It is now in the right form to substitute numbers in and input it into your calculator to find the value of the activation energy.
Worked example: An experiment is carried out at a temperature of 25 °C.
The rate constant = 3.2 x 10-³
R = 8.31 J K-¹mol-¹
ln A = 16.9
Calculate the activation energy for this reaction.
Answer:
lnk = ln 3.2 x 10-³ = -5.74
Ea = R x T (lnA-lnk) = 8.31 x 298 (16.9 + 5.74)
= 56076 J mol-¹
Ea = 56 kJ mol-¹
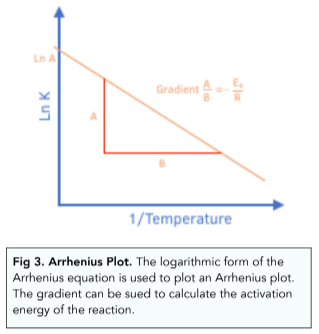
Using a Graph of lnK vs 1/T to Determine the Activation Energy
As shown in the above diagram, you can plot a graph of ln k against 1/ T. This will give a straight line graph with a gradient of -Ea/R.
You can then use the gradient to find out the activation energy and the Arrhenius constant using experimental data.
Worked example: Use the data in the table to calculate the activation energy and Arrhenius constant for the reaction.
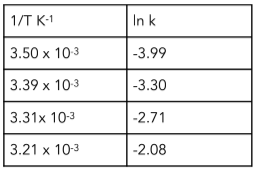
R = 8.31J K-¹mol-¹
Answer:
Step 1: Plot a graph of ln k vs 1/T
Draw a line of best fit. Make it as large as possible so it crosses the y axis.
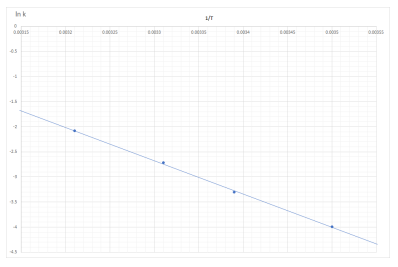
Step 2: Determine the intercept
The intercept, i.e. point where it crosses the y axis is ln A.
Intercept from the graph = -1.65 = ln A
So A = 0.192
Step 3: Determine the gradient
The gradient of the line = Ea/R
Gradient = change in y/ change in x
Use the points (0, -1.65) and (0.0035, -4) for the gradient.
Gradient of the line = (-1.65 -(-4))/ (0.0035-0.00315) = 6.714 x 10³ = Ea/R
Therefore Ea = 6.714 x 10³ x 8.31 = 55795.7 J mol-¹ = 55.8 kJ mol-¹





Still got a question? Leave a comment
Leave a comment