Atomic Structure - Subatomic Particles (A-Level Chemistry)
Subatomic Particles
Atoms
An element is made of one type of atom. Elements are made up of atoms, and each element only has one type of atom. For example, oxygen is only made up of oxygen atoms.
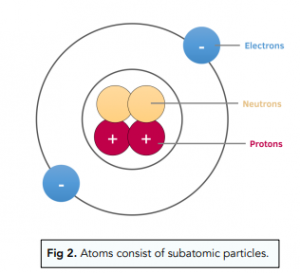
Atoms consist of protons, neutrons and electrons. Atoms are made of protons, neutrons, and electrons, which are types of particle also known as subatomic particles.
Each subatomic particle has a relative mass and relative charge. We use relative masses and relative charges instead of actual values, because the actual mass of these subatomic particles is extremely small. These values are shown in the table below.
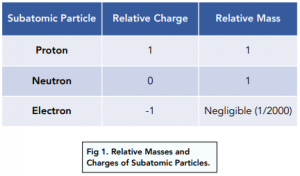
Differences in relative charge result in subatomic particles behaving differently as they travel at constant speeds through electric fields.
A beam of positively charged protons will be deflected towards the negative electrode.
A beam of negatively charged electrons will be deflected towards the positive electrode.
A beam of neutrons will continue in a straight line because neutrons have no charge.
Protons and neutrons are found in the centre of an atom. Atoms are mostly empty space surrounding a very small, dense nucleus that contains both the protons and the neutrons.
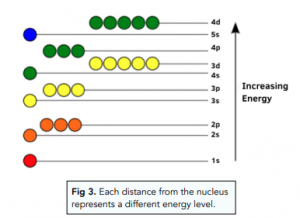
Electrons surround the nucleus. The electrons are found in orbitals which surround the nucleus. Electrons can have different energy levels, and each distance from the nucleus represents a different energy level.
Atomic Models
The currently accepted model of atomic structure has been preceded by other models in the past, which were then modified as new evidence appeared. Throughout history there have been five models of basic
atomic jstructure
- Dalton’s model of the atom. John Dalton suggested that all matter was composed of tiny, indivisible, solid spheres which he called atoms and that different elements where made up of different atoms.
- Thompson’s model of the atom. In 1897 J.J. Thompson first discovered the electron. He suggested that atoms consisted of negatively charged particles embedded in a sea of positive charge, like plums in a pudding, hence the ‘plum pudding model’ name.
- Rutherford’s model of the atom. In 1909, Ernest Rutherford conducted an experiment where he fired positively charged alpha particles through a thin sheet of gold. Most of the particles passed straight through but a small amount were deflected backwards. He therefore proposed that atoms consisted of a tiny, dense, positively charged core or nucleus surrounded by a cloud of negatively charged electrons.
- Bohr’s model of the atom. In 1920 Niels Bohr proposed that electrons could only exist in shells or orbits at different energy levels around the nucleus. When electrons moved from from one shell to another, they emitted or absorbed electromagnetic radiation of fixed frequency. This model was later refined to include sub-shells.
- Chadwick’s model of the atom. In 1932 Rutherford suggested there must exist another particles in the nucleus along with protons, which would otherwise strongly repel each other. James Chadwick later discovered the neutron.
FAQs
The atomic structure in A-Level Chemistry refers to the fundamental make-up of atoms and the subatomic particles that make up the atoms. This includes the protons, neutrons, and electrons.
Subatomic particles in A-Level Chemistry are tiny particles that make up the atoms. These particles include protons, neutrons, and electrons.
Protons in A-Level Chemistry are subatomic particles that are found in the nucleus of an atom. They have a positive charge and determine the atomic number of an element.
Neutrons in A-Level Chemistry are subatomic particles that are also found in the nucleus of an atom. They have no charge and are important for determining the atomic mass of an element.
Electrons in A-Level Chemistry are subatomic particles that orbit the nucleus of an atom. They have a negative charge and are important in chemical reactions and determining the chemical properties of an element.
The protons, neutrons, and electrons make up the atomic structure in A-Level Chemistry by working together to form the complete atom.





Still got a question? Leave a comment
Leave a comment