Atomic Structure - Element Isotopes (A-Level Chemistry)
Element Isotopes
Isotopes
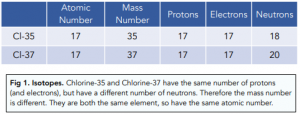
Isotopes are atoms of the same element with different number of neutrons. This means the isotopes have different mass numbers but the atomic number stays the same.
- Physical properties of isotopes are different. Isotopes can have varying physical properties, because mass determines physical properties such as density, boiling and melting point.
- Chemical properties of isotopes are pretty similar. Chemical properties are determined by the number and arrangement of electrons which do not change.





Still got a question? Leave a comment
Leave a comment