Oxidation, Reduction and Redox Equations - Oxidation States (A-Level Chemistry)
Oxidation States
Key Terms
An oxidation reaction is the process of electron loss. Oxidising agents are electron acceptors.
A reduction reaction is the process of electron gain. Reducing agents are electron donors.
An oxidising agent gets reduced by accepting electrons
A reducing agent gets oxidised as it gives up electrons.
Oxidation Numbers
The oxidation state (or number) of an element represents the total number of electrons that the element has either accepted by an element (negative oxidation number) or removed from an element (positive oxidation number), to get to its current form. It shows us the degree of oxidation of an atom or ion in a compound.
Oxidation involves an increase in oxidation state
Reduction involves a decrease in oxidation state
Example: Potassium Chloride
For example, in the reaction between K and Cl:
- K loses an electron to form a positive K+ ion. Its oxidation number increases by 1.
- Cl gains an electron to form a negative Cl- ion. Its oxidation number decreases by 1.
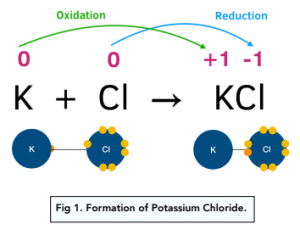
In general, metals like potassium, will form positive ions by loss of electrons with an increase in oxidation state.
In general, non-metals like chlorine, will form negative ions by gain of electrons with a decrease in oxidation state.
Rules for Assigning Oxidation States
You should be aware of the 8 main rules for assigning oxidation states of an element in a compound or ion from the formula.
1. The oxidation state of an uncombined element is zero. This is because it hasn’t been either oxidised or reduced yet.
E.g. the oxidation state of Argon is 0.
2. The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
E.g. NaCl has an overall oxidation state of zero
Oxidation state of Sodium = +1
Oxidation state of Chlorine = -1
3. The sum of the oxidation states of all the atoms in a simple monoatomic ion is equal to the charge on the ion.
E.g. The oxidation state of K+ is +1.
4. The overall oxidation state in compound ions is the overall ion charge.
E.g. The overall oxidation state of NO3- (nitrate ion) is -1.
Oxidation state of Nitrogen = +5
Oxidation state of Oxygen = -2 x 3 = -6
5. Elements that exist as molecules involving identical atoms bonded together have an oxidation state of zero.
E.g. O2 or Cl2 have an oxidation state of zero.
6. The more electronegative element in a substance is given a negative oxidation state. The less electronegative one is given a positive oxidation state.
It is worth to note here that fluorine is the most electronegative element, then oxygen.
7. Some elements almost always have the same oxidation states in their compounds:
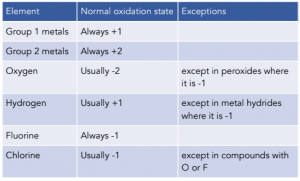
In general, d-block elements (transition metals) can form ions with different charges and hence have different oxidation states in their compounds.
8. Roman numerals can also represent oxidation states in chemical names.
E.g. Iron has an oxidation state of +2 in iron(II) chloride (FeCl2) whereas it has an oxidation state of +3 in iron (III) chloride (FeCl3).
Oxidation is a chemical reaction in which an element loses electrons, resulting in a change in its oxidation state. This change in oxidation state is often accompanied by a change in the chemical properties of the element and can be used to identify which elements are undergoing oxidation in a reaction.
Reduction is the opposite of oxidation and is a chemical reaction in which an element gains electrons, resulting in a change in its oxidation state. Reduction reactions are typically accompanied by a change in the chemical properties of the element and can be used to identify which elements are undergoing reduction in a reaction.
Redox equations are chemical equations that describe the oxidation and reduction reactions that occur in a chemical reaction. These equations can be used to balance redox reactions and to determine the oxidation states of the elements involved in the reaction.
The oxidation state of an element is a number that represents the number of electrons that an element has lost or gained in a chemical reaction. The oxidation state of an element can be used to determine which elements are undergoing oxidation or reduction in a reaction and to balance redox equations.
Oxidation and reduction are opposite chemical reactions that are characterized by changes in the number of electrons that an element has. In oxidation reactions, elements lose electrons, while in reduction reactions, elements gain electrons. The transfer of electrons between elements is what drives the chemical reaction and results in changes in the oxidation state of the elements involved.
The study of oxidation, reduction, and redox equations is important in chemistry because it provides a deeper understanding of the underlying processes that drive chemical reactions. This knowledge can be applied to a wide range of fields, including medicine, biochemistry, and environmental science, and can help researchers to develop new treatments, technologies, and strategies for preserving the environment. By studying oxidation, reduction, and redox equations, we can gain a better understanding of how matter interacts and changes at the molecular level.






Still got a question? Leave a comment
Leave a comment