The Halogens - Group 7, The Halogens (A-Level Chemistry)
Group 7, The Halogens
Introduction to the Halogens
Elements in Group 7 are collectively known as the halogens.
- Group 7 elements are all non-metals and exist as diatomic molecules. In each molecule, two atoms of the element are held together by a single covalent bond. Adjacent diatomic molecules have weak London forces between them.
- Halogen atoms have 7 electrons in their outermost shell. The electron configuration of their outermost shell is always s2 p5. In order to achieve the stable electron configuration of a noble gas they only need to gain one electron to form a 1- ion. As a result, they are very reactive elements.
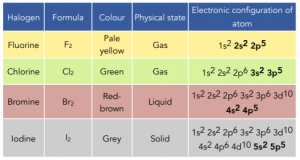
You will need to know the properties of the first 4 halogens:
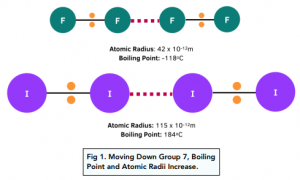
Boiling Point Trend
- As you move down Group 7, the boiling points of the elements increases. As you move down Group 7, the van der Waals forces become stronger as the size of the Group 7 molecules increases. As learned earlier, the bigger the molecule, the more electrons are present therefore the stronger the van der Waals forces. The trend in boiling points is represented by the changes in the physical states of the halogens from gas to solid.
- As you move down Group 7, the melting points of the elements increases. Once again, this is due to increased strength of Van der Waals forces between halogen molecules.
- As you move down Group 7, the volatility of the elements decreases. Volatility refers to the ability of a substance to turn from a solid directly into a gas. Do to the weak Van der Waals forces between diatomic molecules, halogens have relatively low volatility points, and the higher up the group you are, the lower this volatility point will be.
Bond Strength Trend
- Bond energies in diatomic molecules of halogens are relatively low. Low bond energies mean that the covalent bonds in the diatomic molecules are weak and hence easy to break.
- As you move down the group, bond energies decrease. The atomic radius of the elements increases down the group so there is an increased shielding effect. This means that there is a reduced attraction between the nucleus of the halogen atoms in the diatomic molecule and the shared pair of electrons in the covalent bond. As a result, the covalent bond in the the diatomic molecule weakens as you go down the group.
Electronegativity Trend
- As you move down Group 7, the electronegativity of the elements decreases. All halogens are very electronegative with fluorine being the most electronegative element. As you move down the group, the atomic radius of the elements increases and there is an increased shielding effect. This means that there is a reduced attraction between the the halogen atoms and atoms of other elements, forming bonds less readily
Solubility of Halogens
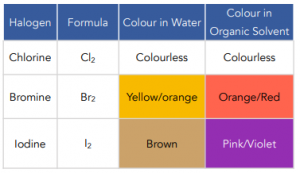
- Halogens are poorly soluble in water. Halogen molecules are nonpolar and hence cannot form hydrogen bonds with water molecules.
- Halogens are soluble in organic compounds. When dissolved in hexane, halogens give solutions of characteristic colours which can be used to identify them.
FAQs
The halogens are a group of chemical elements in the periodic table, listed as Group 7 and include fluorine, chlorine, bromine, iodine, and astatine.
The halogens have a high electron affinity, meaning they easily gain electrons to form negative ions. They are also highly reactive and typically exist as diatomic molecules in nature.
The reactivity of the halogens decreases as you move down the group in the periodic table.
The electron configuration of the halogens is characterized by having seven valence electrons in their outer shell.
The halogens form compounds by gaining one electron to form a negative ion, or by sharing electrons with other elements to form a covalent bond.
The halogens are important in daily life as they are used in many products and processes, such as in the production of halogen lamps, refrigerants, and disinfectants.
Fluorine plays a key role in dental health as it helps to prevent tooth decay by strengthening the enamel on teeth.
Halogens react with metals to form salts, where the metal ion forms a positive ion and the halogen forms a negative ion.
Halogens are commonly used in industry as building blocks in the synthesis of organic compounds, as well as in the production of dyes, plastics, and pharmaceuticals.
No, halogens are not essential to life, but they do play important roles in many natural processes and human-made products.






Still got a question? Leave a comment
Leave a comment