Introduction to Organic Chemistry - E/Z Isomerism (A-Level Chemistry)
E/Z Isomerism
E/Z Isomerism
Stereoisomerism
Stereoisomers are molecules with the same molecular formula but differing positions in space. There are two types of stereoisomerism: E/Z isomerism (which we will cover in more detail in this chapter) and optical isomerism (which you will meet later).
E/Z Isomerism
- E/Z isomerism occurs in alkenes. Alkanes are unsaturated hydrocarbons with C=C bonds and general formula CnH2n.
- E/Z isomerism occurs due to restricted rotation about C=C double. C=C double bonds contain both σ and π bonds. The π bonds arise as a result of the overlap of 2p orbitals from both C atoms in the formation of the covalent bond. The overlap occurs above and below the C atoms, preventing free rotation of the groups about the double bond.
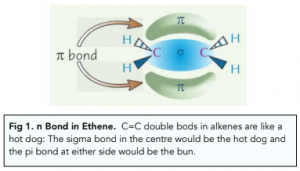
- E/Z isomerism only occurs if the groups bonded to each carbon atom in the C=C bond are different. Due to the restricted rotation about the planar C=C double bond, the position of the groups bonded to the carbons in the double bond cannot be interchanged. Therefore, different isomers exist.
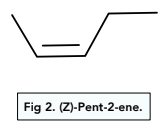
The Z-isomer has the groups with priority (more on this later) together, either above or below the carbon, carbon double bond.
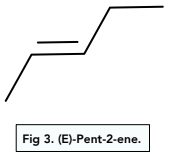
The E-isomer occurs when the groups with priority are on opposite sides of the double bond.
CIP Rules for E/Z Stereoisomers
CIP stands for Cahn-Ingold-Prelog priority rules. These are the rules which determine whether a molecule is an E or a Z isomer.
Even when the C=C in a molecule is next to more than two unique groups, it can show E/Z isomerism.
There are some key rules to remember to work out if a molecule is an E or a Z isomer. These refer to the atom or atoms bonded to the two carbons in the double bond.
- For single atoms, a higher atomic mass gives a molecule higher priority. For example, bromine has a higher atomic mass than hydrogen and so has higher priority.
- For groups of atoms, look at the atom directly bonded to the carbons in the double bond. Whichever carbon is bonded to the atom with the highest atomic mass will have priority. If these are the same (for example if both are carbons), look down the chain.
If the groups with higher priority are on the same side, then it is a Z isomer.
If they are on opposite sides, then it is an E isomer.
Cis-Trans Isomerism
Cis-Trans isomerism is a special type of E/Z isomerism in which both of the carbon atoms of the C=C group have at least one substituent group in common.
Cis isomers will have the equal groups on the same side and trans isomers will have the equal groups on different sides.
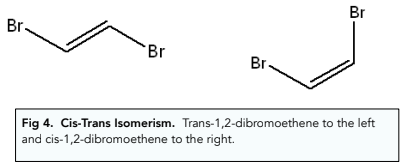
Drawing E/Z Isomers
To draw this form of isomers:
- Draw the longest chain described in the name of the compound. Refer back to chapter 72 if you do not remember the different stems used to name carbon compounds of different chain lengths.
- Number the carbons. The main chain will be numbered in such a way the substituents are given the lowest number as possible.
- Add the functional groups to the carbons described in the name. The carbon to which the functional group is bonded to will be indicated with a number in the name.
- Ensure that the groups are in the correct arrangement across the double bond. In Z isomers the high priority groups will be on the same side and in E isomers they will be on opposite sides.
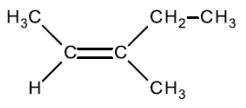
Worked example: 3-methylpent-2-ene exists as E and Z isomers. Draw the structure of Z-3-methlypent-2-ene.
Answer:
1) Draw the C=C and then add the side chains from each atom around it as shown.
2) Identify and draw the groups with the highest masses on the same side of the double bond (Z isomer)
FAQs
Organic chemistry is the study of the structure, properties, and reactions of organic compounds, which are compounds that contain carbon atoms.
In an E isomer, the two highest priority groups (i.e., those with the highest atomic number) on each carbon atom are on opposite sides of the double bond. “E” stands for “entgegen,” which is German for “opposite.”
In a Z isomer, the two highest priority groups on each carbon atom are on the same side of the double bond. “Z” stands for “zusammen,” which is German for “together.”
The E and Z notation is commonly used in organic chemistry to describe the stereochemistry of double bonds. It is important because the different spatial arrangements of E and Z isomers can affect their physical and chemical properties, including reactivity and biological activity.
E and Z isomerism is a type of stereoisomerism, which is a type of isomerism that arises due to differences in the spatial arrangement of atoms in molecules. Steroisomers have the same molecular formula and connectivity of atoms, but differ in their three-dimensional orientation or arrangement. In the case of E and Z isomers, they differ in the orientation of groups around a double bond, which is a type of geometric isomerism.
E isomers have a trans arrangement of substituents on a double bond, while Z isomers have a cis arrangement.
To draw E and Z isomers, follow these steps:
Identify the carbon atoms that are double bonded.
Determine the priority of the groups attached to each carbon atom based on their atomic number. The group with the highest atomic number is assigned the highest priority, and the group with the lowest atomic number is assigned the lowest priority.
Draw a horizontal line between the two carbon atoms to represent the double bond.
Draw the groups attached to each carbon atom on either side of the double bond, with the highest priority group at the top and the lowest priority group at the bottom.
Determine whether the two highest priority groups on each carbon atom are on the same side (Z) or opposite sides (E) of the double bond. If the two highest priority groups on each carbon atom are on the same side of the double bond, it is a Z isomer. If they are on opposite sides, it is an E isomer.
To determine whether a molecule is an E or Z isomer, you need to consider the priority of the substituents attached to the double bond. The substituent with the highest priority should be on the same side of the double bond.
Understanding E/Z isomerism is important in A-Level Chemistry as it helps to explain the stereochemistry of organic compounds and the different properties that result from the different arrangements of atoms in space.
E/Z isomerism affects the properties of organic compounds, such as their physical and chemical properties, as the different arrangements of atoms in space result in different molecular shapes and interactions.
Cis-trans isomerism is a type of stereoisomerism that arises due to the restricted rotation around a double bond or a ring in a molecule. It occurs when two substituents attached to the double bond or ring are different, and their relative positions cannot be interchanged by rotation around the bond or ring.
In cis-trans isomerism, the two stereoisomers are referred to as cis and trans isomers. Cis isomers have the two substituents on the same side of the double bond or ring, whereas trans isomers have the two substituents on opposite sides of the double bond or ring.
Cis-trans isomerism is commonly seen in organic molecules that contain a carbon-carbon double bond or a cyclic structure, such as alkenes, cycloalkanes, and some aromatic compounds. It can affect the physical and chemical properties of the molecules, including their reactivity, biological activity, and solubility, among others.
E/Z isomerism can impact the reaction of organic compounds as the different arrangements of atoms in space can affect the reactivity of the molecule. This is because different isomers can have different orientations of functional groups and different interactions with reactants and products.






Still got a question? Leave a comment
Leave a comment