Introduction to Organic Chemistry - General Formulae (A-Level Chemistry)
General Formulae
Formulae of Organic Compounds
General Formulae
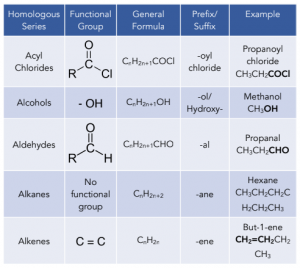
The general formula of a homologous series can be used to figure out the molecular formula of a compound with that particular functional group and n carbon atoms. For example the general formula of alcohols is CnH2n+1OH. If there are 3 x C atoms, there will be (2 x 3) + 1 = 7 x H atoms. The formula will be C3H7OH.
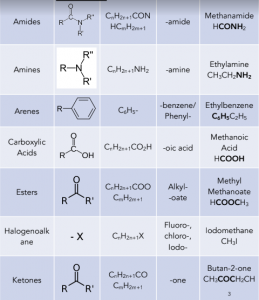
Here you can find a compilation of the main homologous series we will be looking at in the next couple chapters on organic chemistry and their corresponding general formulae.
The letter R represents an alkyl group (general formula CnH2n+1) or hydrogen atoms. The letter X represents a halogen atom.
FAQs
Organic Chemistry is the study of the structure, properties, and reactions of organic compounds, which are compounds that contain carbon.
Common examples of organic compounds include sugars, fats, proteins, and petroleum products.
The general formulae of organic compounds are based on their functional groups, which are specific arrangements of atoms within the compounds. For example, the general formula for alkanes is CnH2n+2.
The difference between an alkane and an alkene is in the presence of a double bond in the latter. Alkenes have the general formula CnH2n and contain a carbon-carbon double bond. Alkanes have the general formula CnH2n+2 and contain only single bonds.
The difference between an alkane and an alcohol is in the presence of a hydroxyl group (-OH) in the latter. Alcohols have the general formula R-OH, where R is an alkyl group, and contain a hydroxyl functional group. Alkanes have the general formula CnH2n+2 and do not contain a hydroxyl group.
The difference between an alkane and a carboxylic acid is in the presence of a carboxyl group (-COOH) in the latter. Carboxylic acids have the general formula R-COOH, where R is an alkyl group, and contain a carboxyl functional group. Alkanes have the general formula CnH2n+2 and do not contain a carboxyl group.
These are some of the basic general formulae of organic compounds that a student studying A-Level Chemistry should know. Understanding these formulae is crucial to understanding the properties and reactions of organic compounds.






Still got a question? Leave a comment
Leave a comment