Acids and Bases - Ionic Product of Water (A-Level Chemistry)
Ionic Product of Water
Ionic Product of Water (Kw)
Kw of Water
Water dissociates to a very small extent:
H2O(l) ⇌ H+(aq) + OH- (aq)
The equilibrium constant Kc is:
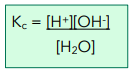
The extent of dissociation is so little that the undissociated water molecules vastly outnumber the ions present. We can therefore assume that the concentration of water molecules remains constant.
If we multiply Kc by the [H2O], we have a new constant. This is known as Kw = ionic product of water.
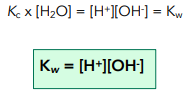
The units of Kw are mol² dm-6.
Value of Kw
The value of Kw is always the same, at a given temperature.
At 298K, 25°C, the value of Kw is always:

In pure water, the [H+] = [OH- ] so that Kw = [H+]²
We can use this information to calculate the pH of pure water at 298K.
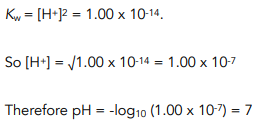
Kw at different temperatures
The value of Kw changes with temperature. The reason for this is because the ionisation of water is an endothermic process.
H2O(l) ⇌ H+(aq) + OH- (aq) ∆H = +ve
When the temperature increases, the equilibrium shifts to the right to oppose the change and reduce the temperature.
The concentrations of H+ ions and OH- ions increase. The value of Kw also increases.
For example, at a temperature of 50 °C, the value of ![]() and the pH of pure water is 6.63.
and the pH of pure water is 6.63.
pKw
The pKw value is calculated in the same way we calculate pH from [H+]
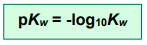
and
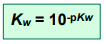
At 298K, pKw = 14.00.
An acid is a substance that donates hydrogen ions (H+) when dissolved in water, while a base is a substance that accepts hydrogen ions when dissolved in water.
The Ionic Product of Water (Kw) is the equilibrium constant for the self-ionization reaction of water, which is the reaction between water molecules to form hydronium (H3O+) and hydroxide (OH-) ions. The value of Kw is constant at 25°C and is equal to 1.0 × 10^-14.
The value of Kw can be used to calculate the concentration of H3O+ and OH- ions in pure water. At 25°C, the concentration of H3O+ ions and OH- ions in pure water is 1.0 × 10^-7 mol/L. This means that in a neutral solution, the concentration of H3O+ and OH- ions is equal.
The pH of a solution is a measure of its acidity or basicity, and is calculated as the negative logarithm of the hydrogen ion concentration (pH = -log[H3O+]). A solution with a pH of 7 is considered neutral, while a solution with a pH less than 7 is considered acidic, and a solution with a pH greater than 7 is considered basic.
The concentration of H3O+ ions directly affects the pH of a solution. As the concentration of H3O+ ions increases, the pH of the solution decreases and becomes more acidic. Conversely, as the concentration of H3O+ ions decreases, the pH of the solution increases and becomes more basic.
No, the concentration of H3O+ ions in pure water at 25°C cannot be greater than 1.0 × 10^-7 mol/L. If the concentration of H3O+ ions exceeds this value, the solution is considered acidic.
The concentration of OH- ions also affects the pH of a solution, but in a different way than the concentration of H3O+ ions. As the concentration of OH- ions increases, the pH of the solution increases and becomes more basic. Conversely, as the concentration of OH- ions decreases, the pH of the solution decreases and becomes more acidic.





Still got a question? Leave a comment
Leave a comment