The Halogens - Reactions with Halogens (A-Level Chemistry)
Reactions with Halogens
Reactivity of Halogens
Oxidising Ability of Halogens
- The reactions of halogens involve the halogen gaining an electron to achieve a full outer shell. Halogens are found in Group 7 of the periodic table meaning that they have 7 electrons in their outermost shell. This means that when they react, they gain one electron to achieve a full outer shell like noble gases.
- The reactivity of halogens decreases as you move down Group 7. Halogens become less reactive as you move down the group as the atomic radius of the element increases which means that the distance between the outer shell of electrons and the nucleus increases. There is a reduced attraction between the nucleus and the outermost shell so it is more difficult to gain an extra electron. This is why the reactivity decreases down the group.
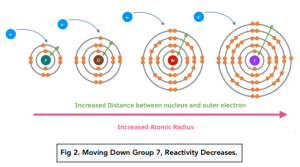
- As the halogens become less reactive down the group, their oxidising ability decreases. Remember that oxidation is a gain of electrons. Halogens become less oxidising as you move down the group as it is more difficult to gain an electron.
- Displacement reactions are a good test for oxidising ability. The oxidising strength of the halogens can be compared by carrying out displacement reactions involving the halide ions. It is worth to note here that a halide in solution will only be displaced by a halogen that is above it in the periodic table.
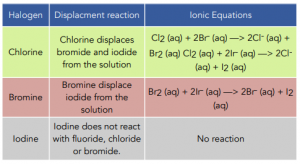
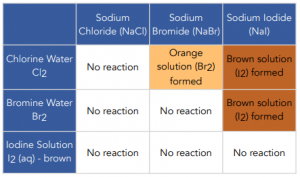
- Displacement reactions can also be used to identify the halogen present in the solution. To identify the halogen present in the solution you look out for the colour change and compare it to the table below
Reactions with Group 1 and 2 Elements
- Halogens react with Group 1 and Group 2 metals to form halide salts. Reactions between halogens and Group 1 and Group 2 metals are typical redox reaction with Group 1 and Group 2 metals being oxidised from an oxidation state of 0 to an oxidation state of 1+ and 2+ respectively.
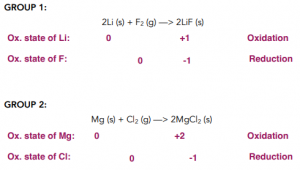
Reactions with Hydrogen
- The trend of reactivity of halogen is illustrated by their reactions with hydrogen. Halogens form covalent bonds with hydrogen to form hydrogen halide molecules. As we go down the group the reaction becomes less vigorous.

- Thermal stability of hydrogen halides decreases down the group. The strength of the covalent bond decreases down the group as the halogen atom increases in size and distance between the atomic nuclei and the shared pair of electrons increases. As a result the temperature required to break these bond decreases.
Halogens are a group of non-metal elements found in Group 17 of the periodic table. They include fluorine, chlorine, bromine, iodine, and astatine. Halogens are reactive and readily form compounds with other elements, including metals and non-metals.
Halogens are highly reactive due to their high electron affinity, meaning they have a strong attraction for electrons. This makes them highly reactive with other elements and they readily form compounds with other elements.
When halogens react with metals, they form salts, also known as halides. The halide ion will have a negative charge and the metal ion will have a positive charge. For example, when chlorine reacts with sodium, it forms sodium chloride (table salt).
When halogens react with non-metals, they form molecules. For example, when chlorine reacts with hydrogen, it forms hydrogen chloride. These compounds are often polar, meaning they have a positive and negative end, and can dissolve in water to form acids.
Halogens have a wide range of uses in chemistry, including in the production of chemicals such as bleach and disinfectants, in water purification, and as refrigerants. They are also used in the production of pharmaceuticals and flame retardants.
The halogen displacement reaction is a reaction in which one halogen is replaced by another halogen. This reaction is driven by the fact that halogens have different electron affinities, and the halogen with the lowest electron affinity will displace the halogen with the highest electron affinity.
Halogen displacement reactions are used in a variety of applications, including in the production of halogenated compounds and in the purification of water. They are also used in the analysis of halogenated compounds, such as determining the presence of halogens in water and soil.
Halogens play an important role in the environment, particularly in the cycling of elements such as chlorine and bromine. They are also used in water purification to kill harmful bacteria and other organisms, and in air purification to remove harmful substances such as ozone-depleting chlorofluorocarbons (CFCs). However, it’s important to use halogens in a responsible manner to minimize any negative impact on the environment.






Still got a question? Leave a comment
Leave a comment