Amount of Substance- The Mole and The Avogadro Constant (A-Level Chemistry)
The Mole and The Avogadro Constant
The Mole and Avogadro Constant
Definitions
The mole is a unit used to measure the amount of substance. One mole of a substance contains as many substance units as there are atoms in 12g of carbon-12. A mole is often written as mol in the shorthand form.
Avogadro’s constant is 6.02 x 1023 atoms. This number is the number of particles (atoms, molecules, ions, electrons) found in one mole.
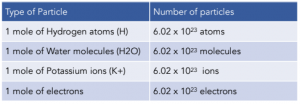
Calculations
It is helpful to learn the following equation as it helps to adjust your calculation depending on which pieces of information you are given:

Let’s look at an example which involves this equation:
Practice Question: How many atoms are present in 0.34 moles of sodium metal?
Number of particles = Number of moles x Avogadro’s constant
Particle = Na atom
Number of moles = 0.34
Avogadro’s constant = 6.02 x 1023
Therefore the number of atoms = 0.34 x 6.02 x 1023 = 2.05 x 1023 Na atoms.
Amount of substance
Mass and Relative Molecular Mass (Mr)
The mass of 1 mole of a substance is the same as the relative molecular mass of the substance in grams.
For example, the relative molecular mass of carbon dioxide (CO2) is 44. This means that 1 mole of carbon dioxide has a mass of 44g.
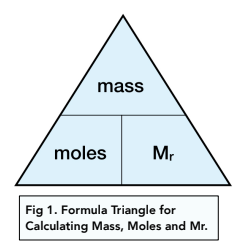
Formula Triangles
When you have an equation that you need to remember, it useful to draw yourself a formula triangle to help you visualise the calculation you need to do.
To calculate the number of moles you can use the following equation:

Here is a formula triangle which helps you visualise the equation. Cover up the piece of information you are not given in the question to help you rearrange the equation.
Example: What is the mass of 2 moles of carbon dioxide CO2 ? (RAM are C = 12, O
Mass of substance = ?
Number of Moles = 2
Mr of substance = 12 + (16 x 2) = 44
Mass of substance = number of moles x Mr = 2 x 44 = 88g
The mole is a unit of measurement in chemistry that is used to express the amount of a substance. It is defined as the amount of a substance that contains the same number of entities as there are in 12 grams of pure carbon-12.
The Avogadro constant is the number of entities in one mole of a substance. It is defined as 6.022 x 10^23 entities per mole.
The mole is important in chemistry because it provides a way to convert between the mass of a substance and the number of entities in that substance. This allows chemists to make accurate and precise measurements of the amount of a substance in a sample.
The Avogadro constant is directly related to the mole. The mole is defined as the amount of a substance that contains the same number of entities as there are in the Avogadro constant.
The Avogadro constant cannot be measured directly. Instead, it is determined by indirect methods, such as X-ray diffraction or electronic spectroscopy.
The Avogadro constant is significant in chemistry because it provides a way to convert between the number of entities in a sample and the mass of that sample. This is essential for making accurate and precise measurements of the amount of a substance in a sample.
In A-Level Chemistry, you can use the mole and Avogadro constant to calculate the number of entities in a sample of a substance, as well as to convert between the mass of a substance and the number of entities in that substance. This will help you understand the concepts of stoichiometry, chemical reactions, and the mole.





Still got a question? Leave a comment
Leave a comment