Alcohols - Alcohol oxidation (A-Level Chemistry)
Alcohol oxidation
Alcohol oxidation
The easiest way an alcohol can be oxidised is by combustion.
When alcohols are burnt in plenty of oxygen, producing a pale blue flame, they get completely oxidised to form carbon dioxide and water.
Oxidation of Alcohols: Oxidation by Cr2O7 2-
Alcohols can be oxidised by a warm solution of potassium dichromate (VII) (K2Cr2O7) mixed with dilute sulfuric acid.
As the alcohol gets oxidised, the C2O7-2 ions in solution become reduced to Cr3+ ions. This causes the solution to change colour from orange to green.
The products of oxidation depends on whether the alcohol is a primary, secondary or tertiary alcohol.
Oxidation of Alcohols: Primary Alcohols
Primary alcohols can be oxidized to form an aldehyde. The aldehyde will have a lower boiling point than the alcohol and can be distilled off.
If however the alcohol is heated under reflux, the aldehyde does not evaporate off and stays in the reaction vessel, which causes it to become further oxidized to form a carboxylic acid.
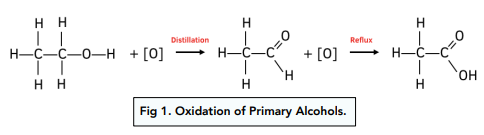
The presence of a carboxylic acid will cause the pH of the reaction mixture to become acidic, which can be tested for with a pH indicator.
Oxidation of Alcohols: Secondary Alcohols
Secondary alcohols are oxidized into ketones. Ketones do not undergo further oxidation.
Oxidation of Alcohols: Tertiary Alcohols
Tertiary alcohols are resistant to oxidation by oxidising agents.
During the oxidation of primary and secondary alcohols the H atom in the -OH group and a H atom on the C atom the -OH is bonded to, are removed to form a C=O bond.
In tertiary alcohols, the C atom the -OH group is bonded to, has no H atom attached to it. Therefore, the C=O cannot be formed.
Tertiary alcohols can only be oxidised by combustion.
Determining Alcohol Structure
The products of oxidation can be used to determine alcohol structure.
For starters, tertiary alcohols can be identified because no colour change will be seen when mixed with potassium dichromate(VII).
If potassium dichromate(VII) solution is mixed with primary or secondary alcohols instead, the solution will change colour from orange to green.
Primary and secondary alcohols can be distinguished by testing the product of oxidation under reflux with 2,4-DNPH.
Secondary alcohols get oxidized to ketones which cause an orange precipitate to form when mixed with 2,4-DNPH.
Primary alcohols on the other hand, get oxidized to carboxylic acids, which do not cause a precipitate to form when mixed with 2,4-DNPH.
FAQs
Alcohol oxidation is a chemical reaction in which an alcohol molecule is converted into an aldehyde or ketone through the removal of one or more hydroxyl groups.
The oxidation reaction of alcohol involves the loss of electrons from the alcohol molecule, resulting in the formation of a carbonyl functional group.
Primary alcohols can be oxidized to aldehydes and then further oxidized to carboxylic acids. The first step of oxidation of a primary alcohol involves the removal of two hydrogen atoms and two electrons from the alcohol group. This forms an aldehyde functional group (-CHO). The aldehyde can then be further oxidized to a carboxylic acid functional group (-COOH) by the removal of additional hydrogen atoms and electrons.
Secondary alcohols can also be oxidized, but they typically form ketones rather than aldehydes or carboxylic acids.
Tertiary alcohols cannot be oxidized under normal conditions because they do not have a hydrogen atom that can be removed.
Yes, primary alcohols can be oxidized. In fact, primary alcohols are the easiest type of alcohol to oxidize. During oxidation, the alcohol functional group (-OH) loses hydrogen atoms and electrons, resulting in the formation of an aldehyde (-CHO) or a carboxylic acid (-COOH) functional group.
The oxidation of a primary alcohol to an aldehyde is an example of a partial oxidation. Further oxidation of the aldehyde to a carboxylic acid is an example of a complete oxidation. The reaction conditions and the oxidizing agent used determine the extent of the oxidation.
The oxidation of primary alcohols to aldehydes can be achieved using mild oxidizing agents such as pyridinium chlorochromate (PCC) or Collins reagent. Stronger oxidizing agents such as potassium permanganate (KMnO4) or sodium dichromate (Na2Cr2O7) can be used to oxidize primary alcohols to carboxylic acids.
Primary alcohols are easy to oxidize because they have a hydrogen atom attached to the carbon atom that is also attached to the hydroxyl group (-OH). This hydrogen atom can be easily removed along with a pair of electrons, resulting in the formation of an aldehyde or a carboxylic acid.
The hydrogen atom is relatively acidic because the electronegative oxygen atom in the -OH group polarizes the C-H bond, making it easier to remove the hydrogen atom. In addition, the carbon-oxygen bond in the alcohol is polar, with the oxygen being partially negative and the carbon being partially positive. This makes the carbon atom more susceptible to attack by an oxidizing agent, leading to oxidation.
Furthermore, the oxidation of primary alcohols to aldehydes is a partial oxidation process that requires less energy and a milder oxidizing agent than the complete oxidation of primary alcohols to carboxylic acids. Therefore, the oxidation of primary alcohols to aldehydes is easier to achieve than the oxidation to carboxylic acids.
Yes, secondary alcohols can undergo oxidation under certain conditions. The oxidation of a secondary alcohol typically leads to the formation of a ketone rather than an aldehyde or a carboxylic acid.
The oxidation of a secondary alcohol involves the loss of hydrogen atoms and electrons from the alcohol functional group (-OH), resulting in the formation of a ketone functional group (-CO-). The reaction conditions and the oxidizing agent used can determine the extent of the oxidation.
Mild oxidizing agents such as pyridinium chlorochromate (PCC) or Collins reagent can be used to oxidize secondary alcohols to ketones. Stronger oxidizing agents such as potassium permanganate (KMnO4) or sodium dichromate (Na2Cr2O7) can also be used, but they are more likely to cause over-oxidation, leading to the formation of carboxylic acids.
Tertiary alcohols cannot undergo oxidation because they do not have a hydrogen atom attached to the carbon atom that is attached to the hydroxyl group (-OH). In oxidation reactions, the hydrogen atom is typically lost from the alcohol functional group (-OH), resulting in the formation of a carbonyl group such as an aldehyde or a ketone.
Since tertiary alcohols lack the necessary hydrogen atom, they cannot form a carbonyl group by oxidation. Instead, they remain stable and do not undergo oxidation. This is because the carbon atom that is attached to the hydroxyl group is fully substituted, meaning it is already bonded to three other carbon atoms.
For example, 2-methyl-2-propanol (tert-butanol) cannot undergo oxidation because the carbon atom attached to the hydroxyl group is already bonded to three other carbon atoms:
(CH3)3COH
In contrast, primary and secondary alcohols can undergo oxidation because they have a hydrogen atom attached to the carbon atom that is also attached to the hydroxyl group, allowing for the formation of a carbonyl group by oxidation.
An oxidizing agent is a chemical species that accepts electrons from the alcohol molecule and facilitates the removal of the hydroxyl group. Common oxidizing agents used in alcohol oxidation include chromic acid (H2CrO4), potassium dichromate (K2Cr2O7), and pyridinium chlorochromate (PCC).
Alcohol oxidation typically follows a two-step mechanism, involving the formation of an intermediate alkoxide ion and the subsequent elimination of a hydroxyl group.
No, alcohol oxidation is a one-way reaction and cannot be reversed to form the original alcohol molecule.
The Alcohol structure can be determined using its systematic name or its common name. The systematic name of an alcohol provides information about its molecular structure and functional groups. The common name of an alcohol is typically based on its source or a historical name, and may not provide as much information about its molecular structure.
To determine the structure of an alcohol using its systematic name, follow these steps:
Identify the suffix “-ol” in the name, which indicates that the molecule is an alcohol.
Identify the longest carbon chain in the molecule, which forms the backbone of the alcohol.
Number the carbon chain so that the hydroxyl group (-OH) has the lowest possible number.
Identify any branches or substituents attached to the carbon chain, and use prefixes such as “methyl”, “ethyl”, “propyl”, and so on to indicate their position and number.
Write the complete name of the alcohol, including the names of any substituents or branches, in alphabetical order.
For example, the systematic name of ethanol is “ethan-1-ol”, which indicates that it has a two-carbon chain with a hydroxyl group on the first carbon. The common name of ethanol is simply “alcohol” or “ethyl alcohol”.
To determine the alcohol structure using its common name, it is necessary to know the molecular formula and the number of carbon atoms in the molecule. For example, “methanol” has one carbon atom, “ethanol” has two carbon atoms, “propanol” has three carbon atoms, and so on. The common names of alcohols can also include prefixes such as “iso-“, “neo-“, or “sec-” to indicate the position or type of branches or substituents.
Alcohol oxidation is widely used in the production of industrial chemicals, fragrances, and pharmaceuticals. It is also an important reaction in the synthesis of carboxylic acids and esters.
When performing alcohol oxidation in a laboratory, it is important to take appropriate safety measures, such as wearing protective gloves and eye protection, working in a well-ventilated area, and avoiding skin contact with the oxidizing agent.






Still got a question? Leave a comment
Leave a comment