Rate Equations - The Rate Equation (A-Level Chemistry)
Rate Equations
The Rate Equations
The rate of reaction depends on the concentration of reactants, the temperature and whether any catalyst present. However these do not equally affect the rate of reaction.
We use the rate equation to explain how rate is affected by the concentration of reactants.
For a reaction in which aA + bB → cC + dD, the general rate equation is:
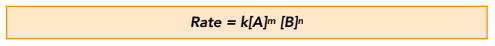
Where:
k = the rate constant
m and n = the order of reaction with respect to the reactants A and B
The unit for rate, r, is ![]() .
.





Still got a question? Leave a comment
Leave a comment