Alkenes - Addition Polymerisation in Alkenes (A-Level Chemistry)
Addition Polymerisation in Alkenes
Polymerisation Reactions
- Polymers are long-chain molecules made from monomers. When several monomers (small molecules) join together and bonds form between them, polymers are formed.
- Alkenes form addition polymers in a polymerisation reaction. The monomers in the reaction would be alkenes which could join together, after the C=C double bond is broken, to form polymers. The addition polymers made from alkenes would be referred to as polyalkenes.
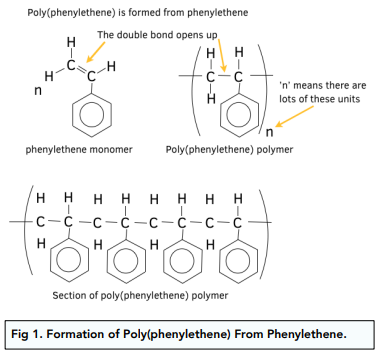
Addition Polymers
Unreactivity of Addition Polymers
- The polyalkenes that are formed only contain single bonds. The addition polymers made from alkenes are saturated compounds because they do not contain double bonds and the main carbon chain is non-polar. This means the addition polymers are unreactive.
Production and Properties of Addition Polymers
- The way we make polymers has changed over time. New catalysts and conditions are being discovered leading to the formation of new polymers like Kevlar.
- Each polymer has different properties. As different polymers have different properties, each one can have a different use and application in real life.
Intermolecular Forces in Polyalkanes
- Intermolecular forces determine the properties of polyalkenes. The long chains of polymers are held together by Van der Waals forces as the chains are non-polar.
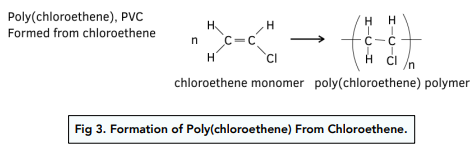
Poly(chloroethene)
- The properties of polymers can be modified using a plasticiser. A plasticiser makes a polymer more flexible by pushing the different polymer chains away from each other. As the chains are now further away, the intermolecular forces between them are weaker and therefore the chains can slide over each other. This causes the polymer to be flexible and bend into different shapes.
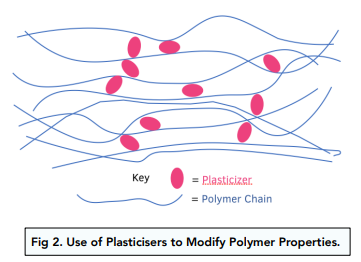
- Polychloroethene (PVC) is used in the production of drainpipes and window frames. PVC is a hard material but it is relatively brittle at room temperature because it has polymer chains that are long and packed closely together.
- If PVC is plasticised it is used in electrical cable insulation, flooring tiles and clothing. When a plasticiser is added to PVC, it makes it more flexible and bendable.
Repeating Units in Addition Polymers
- A repeating unit is portion of the whole molecule that repeats itself several times. A polymer consists of lots of repeating units joined together. The repeating unit has a similar structure to the monomer, except it has the double bond opened up.
Method for Drawing Repeating Units of Addition Polymers From Monomers
- First draw the C=C double bond and the group of atoms attached to each carbon.
- Next replaced the double bond with a single bond and then add an extra single bond coming out of each of the carbon atoms.
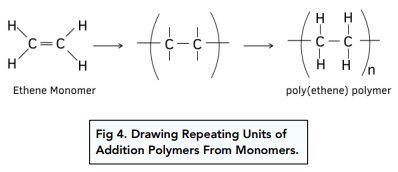
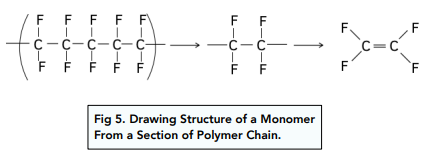
Method for Drawing Monomer Structure From A Section of Polymer Chain
- First look at the entire polymer chain and figure out which section is repeating itself. This will be classed as the repeating unit.
- Once you have identified the repeating unit, add a double bond and that will be the monomer.
IUPAC Rules for Naming Addition Polymers
- Addition polymers are named after the monomers that it is made of. When naming an addition polymer, you add ‘poly’ to the name of the alkene monomer. Remember to add brackets around the alkene monomer, e.g. the addition polymer of ethene would be poly(ethene).
Worked example: Name the polymer below.
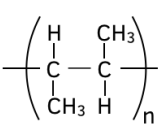
Answer: Poly(but-2-ene)
Addition polymerisation is a chemical reaction in which small molecules called monomers are combined to form large molecules called polymers through the addition of chemical bonds. In alkenes, this reaction occurs between the double bonds of the alkene molecules to form a polymer.
Addition polymerisation in alkenes occurs when the double bonds of the alkene molecules are broken and new single bonds are formed between the carbon atoms of adjacent monomers. This results in the formation of a long chain of carbon atoms, or polymer.
Some examples of addition polymerisation in alkenes include the formation of polyethylene, a common plastic, and polypropylene, another common plastic used in a variety of products.
Addition polymerisation in alkenes has many benefits, including the ability to create a wide variety of polymers with different physical and chemical properties, the ability to produce large quantities of polymer, and the ease of production compared to other methods of polymerisation.
The degree of polymerisation in alkenes is determined by the number of monomers that have reacted to form the polymer. This is often expressed as a ratio, such as the number of monomers per polymer chain.
Yes, the properties of a polymer formed from addition polymerisation in alkenes can be controlled by adjusting the conditions of the polymerisation reaction, such as temperature, pressure, and the type of initiator used. By controlling these conditions, it is possible to create polymers with specific physical and chemical properties for a wide range of applications.






Still got a question? Leave a comment
Leave a comment