Ionisation Energies (A-Level Chemistry)
Ionisation Energies
Ionisation Energy
Ionisation
Ionisation is the process by which an electron is removed from an atom or a molecule. The process is endothermic, because energy is required to break the force of attraction between the electron and the central positive nucleus.
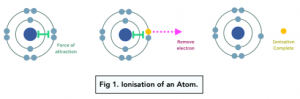
Ionisation Energy
The first ionisation energy is the energy needed to remove 1 electron from each atom of an element in 1 mole of gaseous atoms, to form 1 mole of gaseous ions with a +1 charge.
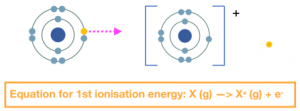
The second ionisation energy is the energy needed to remove 1 electron from each ion of an element in 1 mole of gaseous +1 ions to form 1 mole of gaseous ions with a +2 charge.
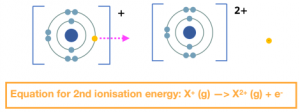
The successive ionisation energy is the energy each time you remove an electron. When talking about successive ionisation energies, we often draw a graph for a particular element of the first ionisation energy, second ionisation energy, third ionisation energy, and so on.

Gas state symbol (g) is used because gaseous atoms are used when ionisation energies are recorded.
Factors affecting Ionisation Energies
Key Definitions
A period in a periodic table is a horizontal row. All the elements in a period have the same number of electron shells.
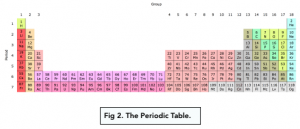
A group in a periodic table is a vertical column. All the elements in a group have the same number of outermost electrons.
The shielding effect is the effect of inner electrons which reduces the pull of the nucleus on the electrons in the outer shell.
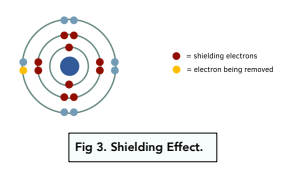
The nuclear charge is a measure of how positive the nucleus is. The higher the atomic number of an element, the more protons it has in its nucleus, and hence the higher the nuclear charge.
The effective nuclear charge is the net positive charge experienced by the outermost electron from the nucleus. It is less than the nuclear charge, because it takes into account the repulsion from inner electrons which provide a shielding effect.
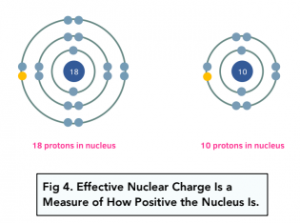
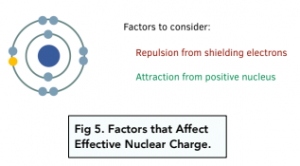
Factors affecting Ionisation Energy
The size of the ionisation energy is determined by the strength of the attraction between the outer shell electrons and the central nucleus. The stronger the attraction, the harder it is to remove the electron and the higher the ionisation energy.
The following factors determine the strength of the attraction between the nucleus and outer electrons:
- Atomic Radius– the higher the atomic radius, the lower the ionisation energy. This is because a higher atomic radius means that the outer electrons are further from the nucleus, and hence the attractive pull from the nucleus is lower.
- Nuclear Charge– the higher the nuclear charge, the higher the ionisation energy. This is because the greater the positive charge of the nucleus, the stronger the attraction for the outer electrons.
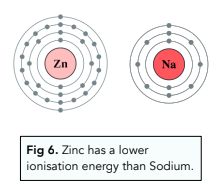
- Number of Inner Shells– the more inner shells present, the lower the ionisation energy. By inner shells, we mean the shells between the outer shell and the nucleus. For example, if the outer electron is in shell 4, then there are 3 inner shells to inner shells repel the outer electrons, lessening the pull towards the nucleus. This effect is called shielding.
For example, Zn has a higher atomic radius and more inner shells than Na, so has a lower ionisation energy. This is despite the fact that Zn has more protons in its nucleus than Na, so there is a greater nuclear charge. The first two factors override the nuclear charge.
Successive Ionisation Energies
Trends in Successive Ionisation Energies
Successive ionisation energies means that more and more electrons are removed, each from an ion that is becoming increasingly positive. We can look at successive ionisation energies to understand shell structure:
- Successive ionisation energies increase between shells. Each time a new shell is broken into, there is a sudden rise in ionisation energy. For example, for sodium, which has one outer electron, there is a big jump in ionisation from the first to second. This is because the second electron is being removed from a shell that is much closer to the nucleus, meaning there is stronger attraction from the nucleus.
- Successive ionisation energies increase within each shell. Each time an electron is removed, even if you haven’t broken into a new inner shell, the ionisation energy increases because you are removing an electron from an increasingly positive ion. For example, for magnesium, which has two outer electrons, the second ionisation energy is greater than the first, because it is harder to remove an electron from an Mg2+ ion than an Mg+ atom, because there is a stronger attraction back.
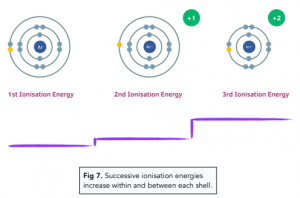
Evidence for Shell Structure
Every time there is a sudden jump in ionisation energy, we can tell that another shell has been entered. So for the diagram below, we can see that there is 1 electron in the outer shell, and then 8 electrons in the next inner shell, and so on.
You can also find out which group of the periodic table the element belongs to by counting how many electrons are removed before the first sudden rise in ionisation energy. For this diagram, we can tell it is a Group 1 element, as it has 1 outer electron.
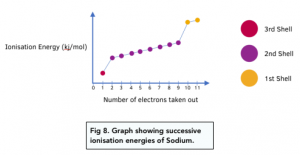
According to this graph for sodium, there is 1 electrons removed before the first sudden rise, then 8 before the next rise and finally 1 electron so the electronic structure for sodium is:
Trends in Ionisation Energies
Re-Cap of Ionisation Energy
To-recap, first ionisation energy is the energy needed to remove 1 electron from each atom of an element in 1 mole of gaseous atoms, to form 1 mole of gaseous ions with a +1 charge.
Above we looked at trends in successive ionisation energies. We can also look at trends in first ionisation energies across both a period and down a group.

Trend Down a Group
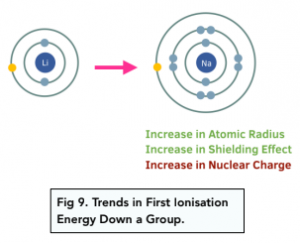
First ionisation energy increases down a group in a periodic table, which means that less energy is required to remove an electron from 1 mole of gaseous atoms. This is due to two factors:
- Atomic radius – the atomic radius increases as you go down a group in the periodic table. As you go down a group, extra electron shell is added each time, so the distance between the nucleus and outermost electron increases. This means that the attraction between the outermost electron and nucleus decreases.
- Shielding effect – the shielding effect increases as you go down a group in the periodic table. As an extra electron shell is added as you go down the group, the number of inner electron shells increases. This decreases the attraction between the outermost electron and the nucleus as there is more repulsion between the electrons.
The nuclear charge actually increases down a group, but the two factor above outweigh the increasing nuclear charge.
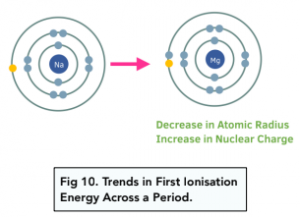
Trend Across a Period
As seen in chapter 60, first ionisation energy increases across a period. This is due to two factors:
- Nuclear charge – the nuclear charge increases as you go across a period. This is because the atomic number increases, and therefore the number of protons in the nucleus increases.
- Atomic radius – the atomic radius decreases slightly as you go across a period. This is a subtle increase because the nuclear charge of the nucleus increases, which means that the nucleus pulls the outer electrons closer to it, which decreases the atomic radius.
There is no change in the shielding effect across a period. All the electrons within the same electron shell are around the same energy level. Electrons are being added to the same outer shell as you go across a period.
Drop between Groups 2 and 3
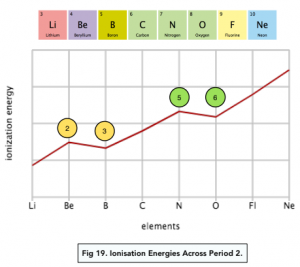
There are a couple of exceptions to the trends across a period.
Firstly, there is a drop in first ionisation energy between Group 2 and 3 elements. For example if we take Beryllium (Group 2) and Boron (Group 3):


We would expect the first ionisation energy of Beryllium to be higher than that of Boron due to the increased nuclear charge and decreased atomic radius.
However as Boron’s outer electron is in a 2p sub-shell rather than a 2s sub-shell, it is at a higher energy level. It is therefore found further away from the nucleus (higher atomic radius) and also experiences more shielding from inner electrons. (i.e. the outermost 2p orbital of Boron is shielded by not only the 1s2 electrons but also the 2s2 electrons).
These two factors override the effect of an increased number of protons in the nucleus on the first ionisation energy, leading to a lower first ionisation energy of Boron than Beryllium.
The drop between Group 2 and 3 provides evidence for electron sub- shells. The change in the usual pattern of ionisation energies can only be proven by the presence of sub-shells.
Drop between Groups 5 and 6
There is also a drop in first ionisation energy between Group 5 and 6 elements. For example if we take Nitrogen (Group 5) and Oxygen (Group 6):
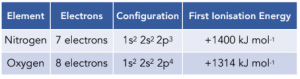
On this occasion, the outer electrons are in the same sub-shell for both elements (2p sub-shell), so there is very little difference in atomic radios or shielding.
The 2p sub-shell has three orbitals:
- In nitrogen, there is one electron in each orbital.
- In oxygen, there are 2 orbitals with one electron each, and 1 orbital with two electrons.
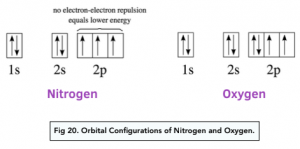
In nitrogen, the electron is removed from an orbital that has only one electron in it, whereas in oxygen it is being removed from an electron from an orbital containing two electrons.
There is more repulsion between a pair of electrons within an orbital so electrons found in such shared orbitals are a lot easier to remove. This is why the first ionisation energy of oxygen is lower than that of nitrogen. This means less energy is required to remove the first electron from oxygen than it is from nitrogen.
FAQs
Ionisation energy refers to the amount of energy required to remove an electron from an atom or ion in the gaseous state.
Ionization energy is a property of an atom or ion, and it is typically listed in a periodic table along with other atomic properties. The ionization energy of an element is usually given as a value in units of kilojoules per mole (kJ/mol) or electron volts (eV).
In a periodic table, the ionization energy typically increases as you move from left to right across a period and decreases as you move down a group. This is because as you move from left to right across a period, the number of protons in the nucleus of the atoms increases, leading to greater attraction between the nucleus and the electrons in the outermost shell. As a result, more energy is required to remove an electron from the atom, leading to an increase in ionization energy.
Similarly, as you move down a group, the atomic radius increases, and the valence electrons are farther from the nucleus. This results in weaker attraction between the nucleus and the outermost electrons, leading to a decrease in ionization energy.
Ionisation energy is important in chemistry as it is used to determine the electron arrangement of elements in the periodic table. It also helps to predict chemical reactions and the behavior of elements in different chemical compounds.
Ionisation energy is typically determined through experiments in which an electron is removed from an atom or ion and the energy required to do so is measured.
The ionisation energy of elements generally increases as you move from left to right across a period in the periodic table and decreases as you move down a group.
Atomic size has a direct impact on ionisation energy. As the size of an atom increases, the ionisation energy generally decreases. This is because the outer electrons are farther away from the positively charged nucleus and are less strongly held.
Ionization energy refers to the amount of energy required to remove an electron from an atom or ion in the gas phase. The first, second, and third ionization energies are the energies required to remove the first, second, and third electrons, respectively, from a neutral atom or ion.
The first ionization energy is the energy required to remove the outermost or valence electron from an atom in the gas phase. This energy is generally represented by the symbol I₁ and is expressed in units of kilojoules per mole (kJ/mol) or electron volts (eV).
The second ionization energy is the energy required to remove the second electron from an ion in the gas phase. This energy is generally represented by the symbol I₂ and is higher than the first ionization energy because it is more difficult to remove an electron from a positively charged ion than from a neutral atom.
The third ionization energy is the energy required to remove the third electron from an ion in the gas phase. This energy is generally represented by the symbol I₃ and is even higher than the second ionization energy because it is more difficult to remove an electron from a doubly charged ion than from a singly charged ion.
Overall, the ionization energy increases as successive electrons are removed from an atom or ion, because the remaining electrons experience greater attraction to the positively charged nucleus. The exact values of the ionization energies depend on the element in question and its electronic configuration.
The main difference between first and second ionisation energy is that the first ionisation energy refers to the energy required to remove the first electron, while the second ionisation energy refers to the energy required to remove the second electron.
Electron configuration has a significant impact on ionisation energy. The electron configuration of an element determines the energy required to remove an electron, with elements having a higher ionisation energy having electrons in a higher energy level or with more shielding electrons.
The ionisation energy is affected by the effective nuclear charge, the atomic radius, and the shielding effect of inner electrons. The effective nuclear charge is the positive charge experienced by an electron, which increases as the number of protons in the nucleus increases. The atomic radius is the distance from the nucleus to the outermost electron, which decreases as the number of protons in the nucleus increases. The shielding effect is the repulsion of inner electrons that reduces the effective nuclear charge felt by outer electrons.
The ionisation energy is an important factor in determining the reactivity of an element. Elements with low ionisation energies tend to be more reactive because they are more likely to lose electrons and form positive ions. Elements with high ionisation energies tend to be less reactive because they are less likely to lose electrons.






Still got a question? Leave a comment
Leave a comment