Aldehydes and Ketones - Reactions to Increase Carbon Chain Length (A-Level Chemistry)
Reactions to Increase Carbon Chain Length
Reactions with Cyanide Ions
The Cyanide Ion
Cyanide ions are very useful in organic synthesis because they can be used to increase the length of the carbon chain.
The negative charge on the carbon in a cyanide ion allows it to act as a nucleophile.
When a cyanide ion is incorporated into an organic molecule, a nitrile functional group forms. This functional group has a carbon atom in it, causing the length of the carbon chain to increase by 1.
Cyanide ions are very toxic. It is unlikely that you will reactions involving them in the lab. If they are carried out, they must be performed in a fume cupboard.
Hydrogen cyanide (HCN) is a weak acid that dissociates in solution to form H+ and CN-. However, HCN is never used as a source of CN ions as it is an extremely poisonous gas.
Potassium or sodium cyanide (KCN or NaCN) are commonly used instead.
Nucleophilic Substitution
Halogenoalkanes can undergo nucleophilic substitution by cyanide ions to form nitriles.
The difference in electronegativity between halogen and carbon atoms, causes halogenoalkanes to have a permanet dipole.
The partial positive charge on the carbon atom leaves it open to attack by nucleophiles.
When a halogenoalkane is heated under reflux with a solution of KCN in ethanol, the halogen atom in the halogenoalkane gets replaced by a cyanide ion to form a nitrile.
Ethanol has to be used as a solvent because water is a stronger nucleophile than cyanide ions and hence would react with the halogenoalkane instead.
The length of the carbon chain of the nitrile will have increased by 1.
The reaction mechanism is shown below:
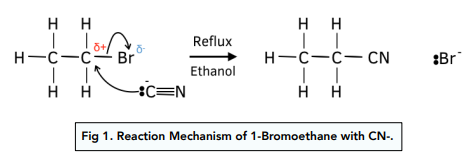
Nucleophilic Addition
Carbonyl compounds can undergo nucleophilic addition by cyanide ions.
At room temperature and pressure, aldehydes and ketones react with potassium cyanide followed by dilute hydrochloric acid.
The dilute acid supplies H⁺ ions which are needed in the reaction mechanism.
This reaction mechanism is important products in organic synthesis, as the carbon chain has increased in length by one carbon atom.
The reaction mechanism for the reaction between propanone and KCN, followed by dilute acid is:
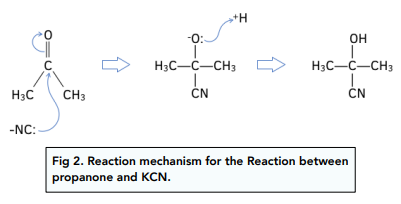
The product is called 2-hydroxy-2-methylpropanenitrile.
Aldehydes and ketones are types of organic compounds that contain a carbonyl group, which is a carbon atom double-bonded to an oxygen atom. Aldehydes have a carbonyl group at the end of the carbon chain, while ketones have the carbonyl group in the middle of the carbon chain.
The difference between aldehydes and ketones in A-Level Chemistry is the location of the carbonyl group in the carbon chain. Aldehydes have a carbonyl group at the end of the carbon chain, while ketones have the carbonyl group in the middle of the carbon chain.
The reactions to increase carbon chain length in A-Level Chemistry include:
Aldol Condensation: An aldol condensation reaction involves the formation of a new carbon-carbon bond between two aldehyde or ketone molecules to form a more complex molecule with a longer carbon chain.
Michael Addition: A Michael addition reaction involves the addition of a nucleophile to the carbonyl group of an aldehyde or ketone, leading to an increase in the length of the carbon chain.
The uses of aldehydes and ketones in A-Level Chemistry include:
Raw Materials: Aldehydes and ketones are used as raw materials for the production of other chemicals, such as alcohols, carboxylic acids, and other organic compounds.
Solvents: Aldehydes and ketones are commonly used as solvents due to their polar nature and ability to dissolve polar compounds.
Perfumes: Aldehydes and ketones are used as fragrances in perfumes and other personal care products.
The properties of aldehydes and ketones in A-Level Chemistry include:
Polar Nature: Aldehydes and ketones are polar compounds due to the presence of the carbonyl group. This makes them soluble in polar solvents, such as water.
Reactivity: Aldehydes and ketones are relatively reactive due to the presence of the carbonyl group, which makes them susceptible to nucleophilic attack.
Boiling Point: The boiling point of aldehydes and ketones increases with the length of the carbon chain. This is due to the increase in intermolecular forces as the molecule becomes larger.
The nucleophilic addition reactions of aldehydes and ketones in A-Level Chemistry involve the addition of a nucleophile to the carbonyl group of the molecule. This reaction can lead to an increase in the length of the carbon chain and the formation of new compounds.
The mechanism of aldol condensation in A-Level Chemistry involves the formation of an enolate intermediate through deprotonation of one of the carbonyl compounds. The enolate intermediate then reacts with the other carbonyl compound through nucleophilic addition.






Still got a question? Leave a comment
Leave a comment