Introduction to Organic Chemistry - Reaction Mechanisms in Organic Chemistry (A-Level Chemistry)
Reaction Mechanisms in Organic Chemistry
Reaction Mechanisms
Introduction to Organic Chemistry – Reaction Mechanisms in Organic Chemistry (A-Level Chemistry) is a core area of study, providing foundational knowledge often applied in Chemistry work experience.
Key Terms
A mechanism describes the steps that a reaction takes as it occurs. Reaction mechanisms are represented by diagrams with the movement of electron pairs as bonds break and form. The movement of electrons is shown by curly arrows.
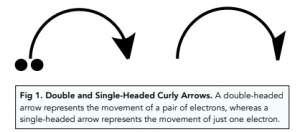
As the diagram shows, the arrow begins from the original position of the lone pair of electrons and point at their final destination when the reaction has occurred. Curly arrows are used very specifically and must be drawn accurately.
Using Curly Arrows: Bond Breaking
If two atoms are joined by a covalent bond, the bond can break either by:
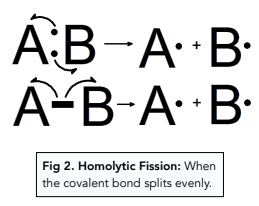
- Homolytic fission – each atom receives one of the electrons in the covalent bond. As one electron moves to each atom, a half curly arrow is used.
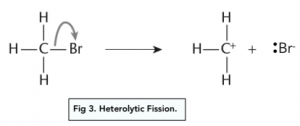
- Heterolytic fission – occurs when the shared pair of electrons move from the covalent bond to one of the atoms from the covalent bond (usually the most electronegative). The atom receiving the pair of electrons will be drawn with a lone pair of electrons and a negative charge. The atom losing the pair of electrons will have a fully positive charge.
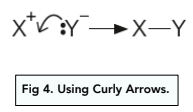
Using Curly Arrows: Bond Making
The curly arrow starts with a lone pair of electrons and points to the atom with which the new bond is made.
Drawing Reaction Mechanisms
As part of the course, you are required to draw mechanisms yourself, using curly arrows.
A few pointers for this are:
1. Draw the displayed formula for each compound involved in the reaction mechanism. Make sure to include all bonds and any relevant lone pair of electrons.
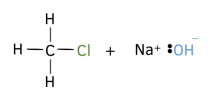
2. Draw curly arrows to show a bond being formed. Ensure that when drawing a a curly arrow to show bonds are being formed it starts starts at a lone pair of electrons or from the centre of another covalent bond, and finishes at an atom.
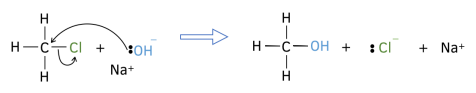
4. Ensure that the charges for the reaction are balance. If it is 1- on one side, it will be 1- on the other side too!
5. Do not include spectator ions in the mechanism. These are the ones which do not change between the two sides of the reactions.
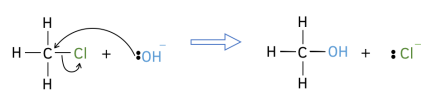
Free Radical Mechanisms
A free radical is an atom or molecule with a single unpaired electron. These form when homolytic fission occurs and the covalent bond splits evenly. The unpaired electron is represented by a bold dot next to the element symbol.
The two main free radical reactions you need to describe are the reaction of alkanes with halogens and the depletion of ozone.
Reaction of Alkanes with Halogens
There are three steps in free radical mechanisms, each with their own equation. It is a chain reaction.
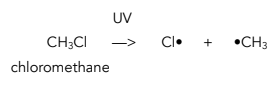
1. Initiation – This is when a free radical is formed by homolytic fission.
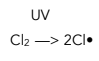
2. Propagation – These reactions produce new free radicals, continuing the chain.
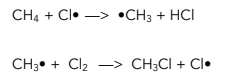
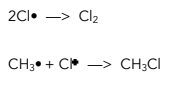
3. Termination – Reactions which do not generate new free radicals, ending the chain reaction.
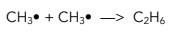
Depletion of Ozone
One specific example of a free radical mechanism is the depletion of ozone in the upper atmosphere.
The reaction for this (which you need to know for your examination) is as follows:
1. Initiation – This is when a free radical is formed by homolytic fission.
2. Propagation – These reactions produce new free radicals, continuing the chain.
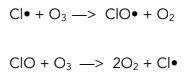
3. Termination – Reactions which do not generate new free radicals, ending the chain reaction.
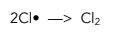
Ozone depletion poses an environmental issue as the ozone layer provides protection from UV rays form the sun. The chlorine free radical is able to break down many hundreds of ozone molecules before terminating by reacting with another chlorine free radical.
Other Reaction Mechanisms
An addition reaction is the formation of a product from two reactants.
An elimination reaction is the removal of a small molecule from a larger one.
A substitution reaction is the replacement of one atom or group of atoms in a molecule for another one.
Hydrolysis is the breakdown of bonds carried out by water.
Oxidation is an increase in the oxygen content or decrease in the hydrogen content of an organic molecule.
Reduction is a decrease in the oxygen content or increase in hydrogen content of an organic molecule.
Over the next couple chapters we will be going over these reaction mechanisms in more detail.
FAQs
Organic chemistry is the branch of chemistry that deals with the study of carbon-based compounds and their properties, structure, and reactions. It encompasses the study of the chemistry of living organisms, as well as the synthesis and properties of a wide variety of products made from carbon-based materials.
Reaction mechanisms in organic chemistry describe the step-by-step process by which chemical reactions occur. They explain how reactants are transformed into products, including the intermediates and the sequence of intermediate reactions that take place. Understanding reaction mechanisms is important because it helps predict the outcome of chemical reactions and provides insight into how to optimize the reaction conditions for maximum yield.
Understanding reaction mechanisms in organic chemistry is important for several reasons. Firstly, it allows you to predict the outcome of a chemical reaction and understand how to optimize reaction conditions for maximum yield. Secondly, it provides a deeper understanding of the chemical principles involved in the reaction and helps to explain why certain reactions occur. Finally, reaction mechanisms can also be used to design new synthetic methods, as well as to improve existing methods, by identifying the rate-determining step in a reaction and making changes to it.
There are several types of reaction mechanisms in organic chemistry, including substitution reactions, elimination reactions, addition reactions, rearrangement reactions, and oxidation-reduction reactions. Each type of reaction mechanism involves a specific set of steps and intermediate compounds that describe the process by which reactants are transformed into products.
Studying reaction mechanisms in organic chemistry involves the use of several techniques, including kinetic studies, spectroscopy, and theoretical calculations. Kinetic studies involve measuring the rate of a reaction as a function of time, while spectroscopy allows for the determination of the structures of intermediates and transition states in a reaction. Theoretical calculations, such as molecular orbital calculations, can be used to predict the energetics of a reaction and to determine the relative stability of intermediate and transition state species.
Understanding reaction mechanisms in organic chemistry has many practical applications. For example, in the pharmaceutical industry, reaction mechanisms can be used to design new drugs and improve existing ones by optimizing the reaction conditions for maximum yield and purity. In the petrochemical industry, reaction mechanisms are used to improve the efficiency of chemical processes and to develop new methods for synthesizing valuable products from petrochemical feedstocks. Additionally, reaction mechanisms play a crucial role in the development of new materials, such as polymers, and in the optimization of chemical processes in the food, cosmetic, and environmental industries.





Still got a question? Leave a comment
Leave a comment