Rate Equations - The Rate Constant (A-Level Chemistry)
The Rate Constant
The Rate Constant, k
How Rate Constant is Related to Rate of Reaction
The rate constant, k, is a number that connects the concentration of reactants in a reaction to the rate of that reaction.
The rate constant is different for every reaction.
The larger the value of k, the faster the rate of reaction.
The value of k can be determined by substituting in the values of rate and concentration of reactants into the rate equation, and then rearranging.
The Units of the Rate Constant, k
The units of k depend on the overall order of the reaction.
Zero Order Reaction
A zero order reaction, rate = k, the units of rate are mol dm−³ s−¹
First Order Reaction
First order – A first order reaction, rate = k[A].
Rearrange the equation above, so we have k = rate / [A]
We know the units of rate are mol dm−³ s−¹ and the units of concentration are mol dm−³.
We now can cancel down to find the units of k.
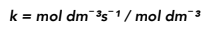
Therefore the units of k for a first order reaction are s−¹.
Second Order Reaction
A second order reaction, rate = k[A][B]
Rearrange the equation above so we have k = rate / [A][B]
Again, cancel down to find the units of k.

Therefore the units of k for a second order reaction are mol−¹dm³s−¹
Third Order Reaction
A third order reaction, rate = k[A][B]²
Rearrange the equation above so we have k = rate / [A][B]²
Again, cancel down to find the units of k.
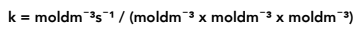
Therefore the units of k for a third order reaction are mol−²dm6s−¹
Worked example:
Results to show the initial rate of reaction with initial concentration of CH3CHO
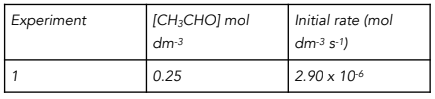
Calculate the value of the rate constant and its units.
The rate equation is: Rate = ![]()
Answer:
Substitute the value of rate and concentration into the rate equation.
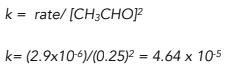
To find the units, write the expression for k without any numbers, just with the units. Cancel common terms.
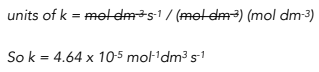
A rate equation is a mathematical expression that describes the rate of a chemical reaction as a function of the concentration of the reactants.
The rate constant, also known as the reaction rate constant, is a measure of the speed of a chemical reaction. It is expressed as a constant value in units of reciprocal time (e.g., 1/seconds).
The rate constant is determined by measuring the reaction rate at various concentrations of reactants and fitting the data to a rate equation.
The rate constant is related to the rate of a chemical reaction by the rate equation. The higher the value of the rate constant, the faster the reaction will proceed.
The value of the rate constant can be affected by factors such as temperature, pressure, and the presence of catalysts or inhibitors.
Yes, the rate constant can change during a chemical reaction, for example, due to changes in temperature or the presence of catalysts or inhibitors.
In A-Level Chemistry, the rate constant is used to describe the speed of chemical reactions and to understand the factors that influence the reaction rate.
Understanding the rate constant is important in A-Level Chemistry as it provides a quantitative measure of the reaction rate and helps to predict the rate of chemical reactions under different conditions. This information is useful in understanding the mechanism of chemical reactions and in the design of chemical processes.






Still got a question? Leave a comment
Leave a comment