Amount of Substance - Further Mole Calculations (A-Level Chemistry)
Further Mole Calculations
Concentration of a Solution
Definitions
The concentration of a particular solution is the number of moles that are dissolved in 1dm3 of a solution. 1dm3 is equal to 1 litre.
The unit mol dm-3 is the unit used to measure the concentration of a solution.
Calculations
The concentration is effectively the number of moles per unit volume. This is the equation used to calculate the number of moles when dealing with concentrations and volumes:
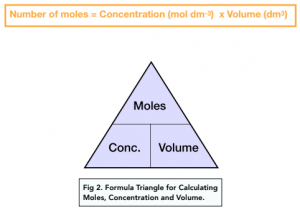
Practice Question: Harry has a beaker containing 25cm3 calcium carbonate solution with concentration 1.5 mol dm 3. What is the mass of calcium carbonate (CaCO3) present in Harry’s solution?
Mr = 40 + 12 + (16 x 3) = 100
Volume of solution in cm3= 25cm3
Volume of solution in dm3= 25/1000 = 0.025 dm3
Number of moles = 1.5 mol dm-3 x 0.025 dm3 = 0.0375 moles
Mass = Mr x number of moles = 100 x 0.0375 = 3.75g
Gas Volumes
Avogadro’s Law
Avogadro’s Law states that equal volumes of gases at the same temperature and pressure contain equal numbers of moles. For example, 10dm3 of oxygen at room temperature and pressure contains the exact same number of moles as there are in 10dm3 of carbon dioxide or any other gas under those conditions.
Definitions
The molar gas volume is the volume of space in dm3 taken up by one mole of a gas at a certain temperature and pressure.
At room pressure and temperature (101.3 kPa and 20oC) the molar gas volume is 24 dm3 mol-1. This value can be simply referred to as molar volume.
At standard pressure and temperature (101.3 kPa and 0oC) the molar gas volume is 22.4 dm3 mol-1.
Calculations
This is the equation used to calculate the number of moles in a volume of gas at room temperature and pressure:
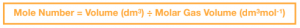
We can use this equation to find out the number of moles of gas produced in a chemical reaction by collecting and measuring the volume of gas evolved using a gas syringe or via water displacement.
Practice Question: Calculate the mass of methane (CH4) contained in a 120cm3 container at the end of a chemical reaction.
Mr = 12 + (1 x 4) = 16
Volume of gas in cm3= 120cm3
Volume of gas in dm3= 120/1000 = 0.12 dm3
Number of moles = 0.12 dm3 ÷ 24 dm3 mol-1 = 0.005 mol
Mass = Mr x number of moles = 16 g mol-1 x 0.005 mol= 0.08g
Water of Crystallisation
Definitions
A salt is type of compound consisting of a lattice of positive and negative ions. It can exist in its pure form or as crystals, with water molecules incorporated in the ionic lattice.
The water of crystallisation is the water molecules that can be incorporated within the crystal structure of a salt.
A salt that contains water of crystallisation as part of its structure is said to be hydrated.
A salt without water of crystallisation is said to be anhydrous.
Formula of Hydrated Salts
A salt will have two different formula depending on whether it is hydrated or not.
For example, the formula for anhydrous copper sulphate is CuSO4, whereas that for hydrated copper sulphate is CuSO4 · 5H2O.
The specific number of moles of water of crystallisation which bind to each mole of salt compound is written after a dot next to the empirical formula for the compound.
Calculations
To find the formula of hydrated salt we have to compare its mass with and without water of crystallisation.
In order to get an anhydrous salt from its hydrated form, we simply have to gently heat it in a crucible until all water is evaporated and mass stays constant.
Practice Question: 3.210g of hydrated magnesium sulphate (MgSO4 · XH2O) are heated to form 1.567g of anhydrous magnesium sulphate. Find the formula of the hydrated salt.
1. Find the number of moles of water lost
Mr of water = (1.0 x 2) + 16.0 = 18 g mol-1
Mass of water lost = 3.210 – 1.567 = 1.643 g
Number of moles of water lost
= mass ÷ molar mass
= 1.642g ÷ 18 g mol-1
= 0.09127 mol
2. Find the number of moles of anhydrous salt
Mr of MgSO4 = 24.3 + 32.1 + (4 x 16.0) = 120.1 g
Number of moles of MgSO4
= mass ÷ molar mass
= 1.567 g ÷ 120.1 g mol-1
= 0.01301 mol
3. Work the ratio of moles of anhydrous salt to moles of water
Moles of anhydrous salt : Moles of water
0.01301 mol : 0.09127 mol
1 mol : (0.09127 ÷ 0.01301)mol
1 mol : 7.015 mol
X can only be a whole number so round off your results
The formula for hydrated magnesium sulphate is MgSO4 · 7H2O.
The amount of substance refers to the quantity of a substance in a sample, often expressed in moles or other units of measurement. This is an important concept in A-Level Chemistry as it allows you to calculate the number of particles in a substance, which in turn helps you to understand its behavior and properties.
A mole is the SI unit of measurement for the amount of substance. It is defined as the number of entities in a sample, such as atoms, molecules, or ions, and is equal to Avogadro’s number, which is 6.022 x 10^23.
To calculate the number of moles in a sample of a substance, you need to know its mass and its molar mass. The molar mass is the mass of one mole of the substance and is often expressed in grams. You can calculate the number of moles by dividing the mass of the sample by its molar mass.
The relationship between moles and mass in a sample of a substance is proportional. This means that as the number of moles of a substance increases, so does its mass. Conversely, as the mass of a substance decreases, so does the number of moles.
The mole concept is a powerful tool that allows you to perform a variety of calculations in A-Level Chemistry. For example, you can use the mole concept to calculate the number of moles of a reactant needed to react with a certain number of moles of another reactant, or to determine the mass of a substance required to produce a specific number of moles.
The mole concept allows you to make quantitative predictions about the behavior and properties of a substance. By using the mole concept, you can make precise calculations about the number of particles in a sample, which in turn enables you to understand its behavior and properties on a molecular level. This makes it a useful tool for both theoretical and practical chemistry.





Still got a question? Leave a comment
Leave a comment