Acids and Bases - The Acid Dissociation Constant, Ka (A-Level Chemistry)
The Acid Dissociation Constant
Acid Dissociation Constant for a Weak Acid (Ka)
Weak acids only partially dissociate to release H+ ions. The equilibrium position lies far to the left. For any weak acid (HA), the dissociation reaction is:
For a general weak acid, HA:
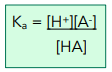
Where [ ] denotes concentration in mol dm-³.
The acid dissociation constant (Ka) is a measure of the extent to which an acid dissociates in solution and therefore its strength. The less an acid dissociates, the smaller the value of Ka. The stronger the acid, the higher the value of Ka.
Using Ka to work out pH of Weak acids
The value of Ka is constant at a specific temperature.
We can use this fact to calculate the pH of a weak acid at a set temperature.
At equilibrium, the [H+] = [A- ], so:
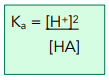
As the degree of dissociation for weak acids is very small, the [HA] at equilibrium can be assumed to be the same as the initial concentration of HA.
If the values of Ka and the initial concentration of HA are known, the concentration of H+ ions can be found by re-arranging the above expression and determining the pH.
Worked Example: Calculate the pH of a solution of 0.02 mol dm-³ of ethanoic acid at 298 K.The value of Ka for ethanoic acid at this temperature ![]()
Answer:
1. Write an expression for Ka
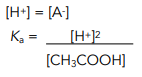
2. Substitute the values into the equation and rearrange the equation to find [H+]

3. Work out pH using [H+]
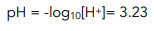
pKa
The pKa value is calculated in the same way we calculate pH from [H+]
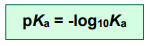
and
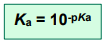
As with pH, the smaller the value of pKa, the stronger the acid.
FAQs
The acid dissociation constant (Ka) is a measure of the strength of an acid in solution. It represents the concentration of hydrogen ions (H+) in solution produced by an acid.
The acid dissociation constant (Ka) is calculated using the equation Ka = [H+][A-]/[HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the conjugate base, and [HA] is the concentration of the acid.
The dissociation constant of a weak acid, denoted as Ka, is a measure of the strength of the acid. It represents the equilibrium constant for the dissociation reaction of the acid in aqueous solution, where the acid donates a proton (H+) to water to form its conjugate base (A-) and a hydronium ion (H3O+). The dissociation constant is defined as:
Ka = [H3O+][A-] / [HA]
where [HA] is the concentration of the undissociated weak acid and [H3O+] and [A-] are the concentrations of the hydronium ion and the conjugate base, respectively, at equilibrium.
The dissociation constant of a weak acid is a measure of its tendency to lose a proton and become ionized in aqueous solution. Stronger acids have higher dissociation constants, meaning that they dissociate more readily and completely than weaker acids. For example, hydrochloric acid (HCl) is a strong acid with a dissociation constant of approximately 1.0 x 10^6, while acetic acid (CH3COOH) is a weak acid with a dissociation constant of approximately 1.8 x 10^-5.
A high value of Ka for an acid means that it is a strong acid, and will readily dissociate into hydrogen ions and its conjugate base in solution.
A low value of Ka for an acid means that it is a weak acid, and will only partially dissociate into hydrogen ions and its conjugate base in solution.
The acid dissociation constant (Ka) is directly related to the pH of a solution. The stronger the acid, the lower the pH, and the higher the Ka value.
The acid dissociation constant (Ka) affects the behavior of an acid in solution by determining how readily the acid will dissociate into hydrogen ions and its conjugate base. Strong acids will dissociate readily and produce solutions with a low pH, while weak acids will only partially dissociate and produce solutions with a higher pH.
The acid dissociation constant (Ka) can be used in practical applications, such as determining the strength of acids and predicting their behavior in chemical reactions. It can also be used to design and optimize chemical processes, such as titrations and buffer solutions.
Yes, the acid dissociation constant (Ka) can be used to compare the strengths of different acids. The higher the Ka value, the stronger the acid.
The acid dissociation constant (Ka) is directly related to the basicity of a conjugate base. The stronger the acid, the stronger its conjugate base will be as a base.
No, the acid dissociation constant (Ka) is specifically used to describe the behavior of acids in solution and cannot be used to predict the behavior of bases. To describe the behavior of bases, the basic dissociation constant (Kb) is used.






Still got a question? Leave a comment
Leave a comment